Fig. 1
Radioactivity distribution within the patch (a). 166Ho-impregnated patch for skin cancer treatment. The size and shape can be easily adjusted according to those of cancers or precancerous lesions (b). Radioactivity within the patch is homogeneously distributed, which was confirmed by autoradiography
The radiation dose from the 166Ho-Patch was calculated by a computer simulation using the EGS4 code system. The effective penetration length of the beta-particles was approximately 2 mm from the surface. The dose rate in a target at a 0.5 mm axial distance from 10 mm size disc shape radioactive patch was 1.248 cGy/s per 37 MBq, which enables the delivery of 40 Gy in less than 1 h when the initial activity of the source is 37 MBq. Based on this simulation data, optimal radiation absorption dose according to the size and shape of the radioactive patch can be calculated (Lee et al. 1997).
However, the preparation methods could be modified using various materials, and accurate radiation absorption dose to the lesions should be calculated.
2.2 Experimental and Human Clinical Trials
The first animal and clinical study for the use of β–-emitting radioactive patches in treatment of skin cancer was done by Lee et al. (1997) and by Chung et al. (1998, 2000). Lee et al. (1997) designed the 166Ho which is a beta-emitting radionuclide impregnated in a polyurethane patch and applied to 10 mice with SCC or AK. The patch was simply applied over the surface of the lesions for 1 or 2 h, which could deliver approximately 42–45 Gy at the tumor surface. All mice showed complete tumor destruction after 1 and 2 weeks of application, but radiation dermatitis or ulceration was seen in all mice, which gradually cleared. Human studies are being conducted in patients with superficial SCC and BCC lesions (Figs. 2, 3, 4), Bowen’s disease (Fig. 5), AK, and Kaposi sarcoma (Fig. 6).
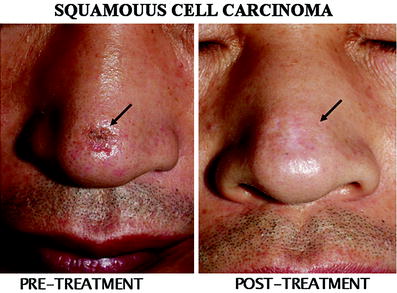
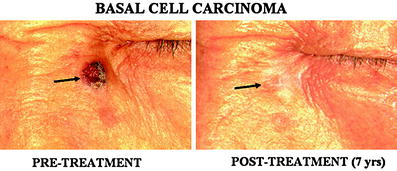
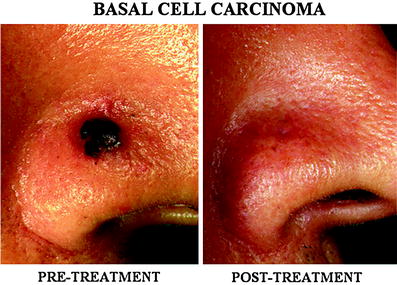
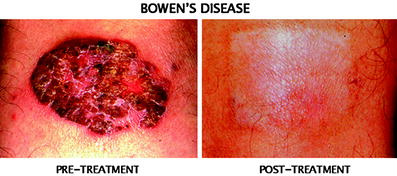
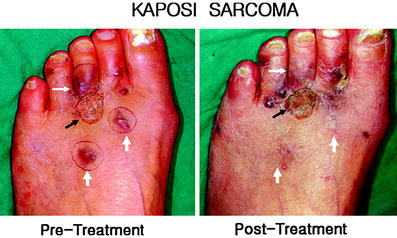

Fig. 2
A superficial squamous cell carcinoma treated with the skin patch. A superficial SCC lesion in the dorsum of the nose (arrow) was completely cured by single session of treatment with total radiation absorption dose of 50 Gy. Mild hypopigmentation can be seen at the treated site

Fig. 3
A basal cell carcinoma (BCC) in the face. A 1.5 cm size BCC (arrow) was successfully treated by single session of treatment. Evidence for tumor recurrence or residual lesion was not seen 7 years after the treatment

Fig. 4
A pigmented basal cell carcinoma (BCC) in the nose. A relatively thick pigmented BCC (estimated depth of the tumor was approximately 4 mm) in the nose was completely cured without residual scar

Fig. 5
A large basal cell carcinoma (BCC) in the thigh. A large 8 × 4 cm size superficial BCC was successfully treated by single treatment using the patch with excellent cosmetic result. Skin graft is usually required if the lesion is to be surgically removed, however, this technique does not require skin graft

Fig. 6
Kaposi sarcomas developed after renal transplantation. Multiple Kaposi sarcomas were developed after renal transplantation. Previously, excision with skin graft was performed (black arrow) with post-surgical scar. Recurrent Kaposi sarcomas were treated with the skin patch (white arrows) without scar or deformity
Approximately a total of 35 Gy of radiation absorption dose was delivered for Bowen’s disease, AK, and Kaposisarcoma; but over 50 Gy for SCC and BCC were delivered through the 166Ho patch when applied for 30 min to 1 h. Chung et al. (2000) treated eight patients with 29 sites of Bowen’s disease with this 166Ho patch. Lesion size ranged from 0.5 to 7.2 cm. Patches were applied for 30–60 min for a total surface radiation dose of 35 Gy. All lesions showed total clearance with acute radiation reaction such as desquamation, erythema, or ulceration within 1–2 weeks after therapy, and all acute radiation reactions healed with minimal fibrosis within 1–2 months. The only complication was hypopigmentation at the treated sites. Complete response was seen in all Bowen’s disease, AK, and Kaposisarcoma. However, tumor thickness over 3 mm needs higher radiation dose or retreatment after first trial due to short range of penetration of the beta-particles (Fig. 7). Therefore, success rate in invasive SCC or BCC depends on the tumor thickness and invasiveness. Usually, tumors with over 3 mm thickness cannot be cured by single session therapy as seen in Fig. 7.
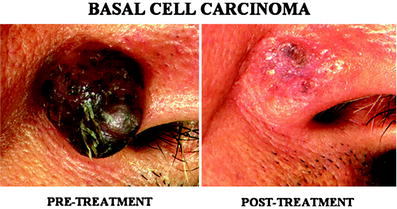

Fig. 7
Partial response in a patient with a large basal cell carcinoma. A large, approximately 1.5 cm size thickness was treated, however, residual lesion can be seen due to the limitation of the short penetration length of the beta-particles. Tumors thicker than 3 mm need multiple sessions of treatment or surgical removal is more preferable
The advantage of this modality is that skin graft after surgical removal is not necessary, which provide more favorable cosmetic results, therefore multiple lesions can be treated without skin grafts as seen in Fig. 6.
Since then, other centers have developed other β–-emitting radioactive patches such as 188Re (Jeong and Chung 2003; Jeong et al. 2003), 32P (Salgueiro et al. 2008a, b, 2009a, b; Pandey et al. 2006, 2008), and 90Y (Mukherjee et al. 2002, 2003) to treat for skin cancer.
Jeong et al. (2003) developed a simple method to produce 188Re patch and applied this radioactive patch to mouse skin cancer and sarcoma model. All skin cancer showed disappearance after 7 days, but sarcoma group showed regrowth after 4 weeks. The authors attributed this to the more thick sarcoma compared to skin cancer. The advantages of 188Re patch are the 188Re produced by 188W/188Re generator which is available for easy access, ease of preparation of 188Re-labeled paper, high-energy transfer (Emax 2.1 MeV), and has a relatively short half-life (16.7 h). The physical properties of various β emitters are seen in Table 1 (modified from Jeong and Chung 2003). The disadvantages of 188Re in radioactive patch application are that although it has a high-energy transfer, it also has a very small penetration depth. Jeong et al. (2003) provided dosimetry data and showed that 57 % of the dose from a 188Re-labeled paper (1 cm diameter, 37 MBq, 1 h) is transferred to 0–1 mm, and that 24 % of the dose is transferred to 1–2 mm, with a radiation treatment of 37 Gy for 0–1 mm depth, and 15 Gy for 1–2 mm depth. These data suggest that 188Re patch would be suitable for lesions that are up to 2 mm thick.
Table 1
Physical properties of β emitters used for patch therapy (modified from Jeong and Chung 2003)
Isotope | T1/2 (days) | Emax (MeV) | Maximum range in water (mm) |
|---|---|---|---|
188Re | 17.0 | 2.11 | 10.8 |
90Y | 2.67 | 2.28 | 12.0 |
166Ho | 26.9 | 1.84 | 9.0 |
32P | 14.3 | 1.71 | 8.7 |
Salguerio et al. (2009b) has developed 32P imbedded patches and studied the efficacy of these patches on animal models with papilloma, keratoacanthoma, and normal skin. The mice were irradiated with either one dose of 40 or 60 Gy, or two sessions of 40 or 60 Gy, and compared with mice with no tumor who received either 40 or 60 Gy. Results showed that tumor size was significantly reduced regardless of the patch administration method, but increased dosage showed higher regression of the tumor. Acute radiation reaction such as erythema, dermatitis, ulceration was seen, but healed during follow-up. Another animal study by the same group (Salgueiro et al. 2009b) studied the effect of 32P patch on melanoma tumor model. The 37 MBq 32P patch was applied twice in an interval of 5 days and tumor growth was compared with the control group. 32P radioactive patch significantly delayed the growth of melanoma in mice, but was not sufficient for complete remission. The group suggested because 32P deposits 99 % of its radiation to within 3 mm, this patch can be used in treatment of melanomas smaller than 2 mm, and combined therapy for lesions larger than 3 mm. Pandey et al. (2006, 2008) also developed a 32P patch for skin cancer therapy. Animal studies with melanoma showed that their patch deposited 10 Gy/hr, and two sessions from 37 to 74 MBq sources at 3-day intervals was sufficient to put the melanoma into remission.
Mukherjee et al. (2002) developed 90Y skin patches and evaluated their effect on fibrosarcoma tumor models. The patches were impregnated with approximately 37 MBq and 74 MBq of 90Y-ferric hydroxide macroaggreggates (90Y-FHMA), and administration of the patches was done when the tumors were approximately 7 mm. The patches delayed the growth of the lesion, but tumor did not completely regress. The group repeated the procedure when the lesion was approximately 2 mm and used a fractionated dose of 92.5 MBq each three times at 7-day intervals. This resulted in the regression of the tumor.
The uses of radioactive patches are not limited to skin cancer. Previous studies have used 32P-coated stents to reduce or delay coronary stent re-stenosis (Hansen et al. 2001; Kay et al. 2001; Kim et al. 2001; Serruys and Kay 2000; Watanabe et al. 1999), and recently a group has developed a plastic stent impregnated with 125I to treat unresectable common bile duct malignancies (Liu et al. 2007, 2009a, b). However, these 32P-coated coronary artery stents are not without problems. Too little radiation can cause re-stenosis as low radiation will stimulate smooth muscle cell proliferation (Weinberger et al. 1996), and excess irradiation can cause aneurysm formation (Bole et al. 1975). One major unsettled question in the clinical uses of these radioactive coronary artery stents is the so-called “candy wrapper effect”, where the stent ends are narrowed due to endothelialization. Further studies are needed to develop radioactive stents to overcome this problem (Serruys and Kay 2000).
Other studies by Won et al. (2002, 2005) have used 166Ho covered self-expandable metallic stents, instead of 125I covered stents for potential treatment and intraluminal patency for common bile duct and esophageal cancers as a combination treatment method, covered self-expandable metallic stent insertion and intraluminal brachytherapy with high-energy radiation from radionuclides. Non-radioactive self-expandable metallic stent implantation is being used for the palliative treatment of inoperable patients with malignant biliary and esophageal stricture, however, ingrowth into the stent lumen or overgrowth over the stent is the major limitation of this treatment modality. On the other hand, 166Ho covered self-expandable metallic stent might be more effective due to its additional intraluminal brachytherapy effect which may prevent from tumor ingrowth into the stent lumen and overgrowth.
In conclusion, within the past two decades, there has been a growing interest in the development of simple, safe, and effective ways to treat cancerous and precancerous skin lesions selectively with minimal damage to the surrounding normal cells. Many skin malignancies are radiosensitive, and β-emitting particles have many advantages over conventional external X-rays in selectively treating these lesions.
References
Archambeau J, Pezner R, Wasserman T (1995) Pathophysiology of irradiated skin and breast. Int J Radiat Oncol, Biol, Phys 31(5):1171–1185CrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree