Fig. 1
At the center is the rheumatologist. Often it is artistic work to juggle with antirheumatoid drugs. Sometimes a drug fails or the whole show ends up as a complete flop. If he recognizes the attractive help by RSO throwing Yttrium-90, Rhenium-186, or Erbium-169 to him, he is able to enhance the quality of his art work. In the interest of noninvasive treatment the orthopedic surgeon stands aside, but he may also have his scene. If all the actors give a combined performance, the applause of the patient will be great
In general it is to be said that the earlier in the course of the disease RSO is performed, the better. The most favorable results in RA are achieved at Steinbrocker stages I and II. But even in advanced stages, RSO makes a positive impact on joint function and quality of live definitely. The clinical efficacy found after RSO in RA patients varied from 42 to 92 % in the prospective and 61–80 % in the retrospective trials (Klett et al. 2007).
2.2 Osteoarthrosis (Degenerative Joint Disease) and Osteoarthritis
It is important to make this distinction: Osteoarthrosis is no indication for RSO, only for OA. Osteoarthritis is a term reflective of clinical practice as a result of inflammatory symptoms in an arthritic joint (Fassbender 1994).
2.2.1 Osteoarthrosis
In osteoarthrosis, the balance between stress-bearing capacity and actual stress for the joints is disturbed. With age, the prevalence of the disease increases though age per se is not causative. Predisposition is not created by cartilage of primarily inferior quality, but by unphysiological stress, e.g., bad posture, posttraumatic axial deviation, ligamentous instability, condition after meniscectomy, occupational, or sport overuse of joints and overweight. In some respects, the pathogenesis of osteoarthrosis is yet unknown (Mödder 1995a).
2.2.2 Osteoarthritis (Activated Arthrosis)
Osteoarthritis (OA) is usually classified as primary (idiopathic) or secondary to metabolic conditions, anatomic abnormalities, trauma, or inflammatory arthritis (Sharma and Kapoor 2007). Or in other words: “Osteoarthritis, the most common human joint disease, is of diverse aetiology and obscure pathogenesis and is a dynamic, but slowly progressive, noninflammatory degenerative disease of cartilages and other tissues of joints, primarily in older individuals with intermittent, inflammatory episodes” (Mankin et al. 1985).
Especially, in respect to RSO, attention has to be focused on the synovium. For example, it is a contentious issue if the initiating moment of arthrosis may lie in the synovial membrane (for the majority of scientists, it is more likely the chondrocyte playing the important role). The main counterargument is that cartilage has no nerves and thus cannot inflict pain being the most serious problem of the patient. The good results of RSO in OA might lead to paradigm change in future (Mödder and Mödder-Reese 2012). That in a certain number of references RSO provides better results in RA than in OA (Kresnik et al. 2002) may be partially due to the fact that rheumatologists refer patients with rheumatic diseases earlier than orthopedics referring patients with more severe joint destruction or after surgical interventions.
Recent studies have attempted to identify specific features of OA that are responsible not only for pain presence but also for severity. Bone and synovium are emerging as potential sources of pain (Hill et al. 2001).
In osteoarthritic synovial tissues, low-grade inflammatory processes occur that contribute to disease pathogenesis, and clinical symptoms and signs in joints (e.g., joint swelling, effusion, stiffness, and occasionally redness) clearly reflect synovial inflammation (Poole et al. 2001).
Arthroscopy has demonstrated localized synovial proliferative and inflammatory changes in up to 50 % of patients with knee OA. Proteases and cytokines produced by activated synovium have been suggested to accelerate deterioration of contiguous cartilage lesions (Lindblatt and Hedfors 1987; Ayral et al. 2005).
The liberation of inflammation mediators (lysosomal enzymes, proteoglycans, and calcium hydroxyapatite crystals) from cartilage attrition and degradation, detritus, and phagocytosis plus mechanical stimulation lead to irritation of the synovium. This reactive or secondary synovitis—with proliferative, frequently villous, pannus-like new connective tissue formation—turns the clinically “silent” arthrosis into the “activated arthrosis” or “osteoarthritis” with painful limitation of motion, often associated with joint effusion. Lymphocytic infiltrations in the stroma of the villi suggest the processes of antigen presentation and antibody formation and may bear witness to the triggering of immunological mechanisms also within the context of OA. “Inflammation transforms the arthrotic into an arthritic” (Fassbender 1994).
Recurrent episodes may lead to fibrosis and retraction of the capsule with increasing stiffness of the joint and contractures. This additional damage of capsule, ligament, and muscle (apparatus) is called “decompensated arthrosis”.
Important: Cartilage has no nerve endings and vessels itself and therefore should not be a source of pain. This is a major reason for the well-known poor relationship between the extent of morphological changes (radiologic or pathologic) and clinical problems like pain (Dieppe 1994). Pain confined to the joints correlates somewhat more closely with findings on soft tissue scintigraphy (Mödder 1995a, b).
2.2.2.1 Osteoarthritis of Finger joints
Figure 2 demonstrates the typical X-ray pattern of so-called “finger polyarthrosis”. Involvement of the DIP joints is called Heberden-Finger polyarthrosis, involvement of PIP joints Bouchard-Finger polyarthrosis. The disease is traditionally regarded as degenerative. But this view has to raise too many doubts. Because extent and frequency of movements of the finger joints increase in proximal direction from DIP to PIP to MCP. Assuming a degenerative cause, the MCP should be involved most. But this is not the fact. Inflammatory MCP joints are on the contrary strongly suspected of being rheumatic. Under biomechanical aspects also, Rhizarthrosis cannot be predominantly explained as degenerative disease. Right-handers often have more symptoms at the left joint. Do the finger joints in Fig. 2 look like worn tyres? Even more evidence presents erosive finger polyarthrosis. Patients may get even more pain and destruction than with progressive RA. There seems to be an overlap between RA and OA. Principally, in soft tissue scintigraphy you can hardly tell the difference between OA and RA. Only the pattern of the inflamed joints permits correct diagnosis.
Because of limitations of satisfying surgery in DIP and PIP joints, finger polyarthrosis RSO moves in the foreground of therapeutic options (Mödder 2006; Rehart et al. 2007).
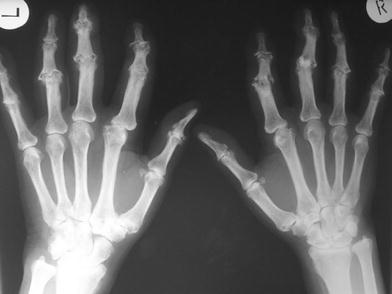

Fig. 2
Osteoarthritis (OA) of the finger joints (see text)
Among different therapeutic measures for activated arthrosis (=OA) the indication for radiosynoviorthesis may be considered in principle.
2.2.2.2 Osteoarthritis of the Knee Joint
Osteoarthritis of the knee joint is the most common form of arthritis in synovial joints. It is characterized by progressive loss of cartilage with periarticular bone remodeling (osteophytes), leading to pain, disability, and handicap in aging populations.
When the subchondral membrane is destroyed—by osteoarthritic progression or after arthroscopy with washout or joint debridement—bone marrow edema with raised pressure in subchondral bone may occur, the most serious source of pain in severe OA, best seen with magnetic resonance imaging (MRI).
Sometimes underestimated additional possible anatomic sites of pain generation in OA are muscle pain, stretching of capsule, ligament insertion strain, tendon insertion, and elevation of periosteum. Recurrent episodes may lead to fibrosis and retraction of the capsule with increasing stiffness of the joint and contractures. This additional damage of capsule, ligament, and muscle (apparatus) is called “decompensated arthrosis”. The rather good effects of RSO in OA (i.e., knee joint) will be missing if mechanical problems as severe instability and axe deviation are predominating (Fig. 4).
Synovial membrane inflammation may play a critical role in disease process and it is likely that at least low-grade synovitis is present in most patients with symptomatic OA (best seen by soft tissue scintigraphy).
The management of knee OA aims at pain including intraarticular application of corticosteroids. The reason for the improvement by this agent is the effect on synovitis. Therefore, this method may be regarded as a prognostic test for RSO: if intraarticular applied cortisone brings pain relief to the patient for a few days or weeks, then RSO usually will be effective for significant longer period. This strategy is required by the Guidelines of the German Society for Rheumatology.
“The synovitis of OA has been described by many authors, some of whom suggest that it has a major role in the disease pathogenesis, implying that some forms of OA are as much an ‘inflammatory’ as a degenerative form of arthritis” (Dieppe 1994).
Very often, one compartment is involved. If medial: varus deformity, if lateral: valgus deformity. Retropatellar arthrosis results from involvement of the patellofemoral joint.
Clinical findings of activated knee arthrosis
Pain: going downstairs (often only backward possible) is usually more arduous than going upstairs; only short walks are tolerated, there is often pain at rest (e.g., crossing the legs). There may be swelling or effusion, resting positions of comfort must be found and diminished joint function might be present.
2.3 Pigmented Villonodular Synovitis
PVNS is a very rare disorder (incidence: 2 cases per one million) characterized by a slowly progressive benign neoplastic proliferation of synovial tissue leading to damage of the cartilage. Metastases have as yet not been observed. With 75 %, the knee is the most common location. Mostly, PVNS is monoarticular, but in few cases polyarticular involvement has been described. The histological diagnosis is established after arthroscopic examination or other surgical procedure. The abundant synovitis may be removed arthroscopically, but the reported relapse rates range between 8 and 46 %. 6 weeks after surgery—when the extensive iatrogenic wounds inside the joint cavity are healed and no leakage would happen—RSO should be performed. A 100 % success rate was reported for this combination of surgical synovectomy and RSO in 11 patients (Kat et al. 2000). A meta-analysis demonstrated 77.3 ± 25.3 % favorable results (Kresnik et al. 2002) (Fig. 3).
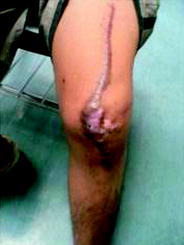

Fig. 3
Boy after 3x surgery because of PVNS (see text). Also because of extreme keloid the knee became stiff. RSO is not yet established in Malaysia, where the boy lives
3 Contraindications
Absolute contraindications
Pregnancy
Breast feeding
Local skin infection
Actual rupture of popliteal cyst (Baker’s cyst).
Relative contraindications
RSO should only be used in children and young patients (<20 years) if the benefit of treatment is likely to outweigh the potential hazards. But RSO is routinely applied in hemophilic children (see “Radionuclide Therapy for Bleeding Joints in Hemophilia” in this volume).
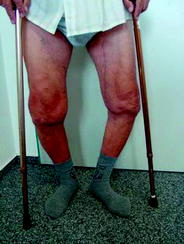
Extensive joint instability with bone destruction (Fig. 4).

Fig. 4
In this patient a great problem is the severe varus axe deviation of both legs. But the main reason for refusing RSO is severe instability, visible because of the crutches
4 Side Effects
Adverse side effects are uncommon and in almost all cases acceptable in view of the benefit of the therapy. A temporary increased synovitis will turn into rapid relief by local application of ice.
A nationwide survey in Germany has shown that RSO with all nuclides currently used in RSO is associated with complications only in about 1:1,000 cases (Kampen et al. 2006).
A transient radiogenic effusion is seen in 2 % of patients (Gratz et al. 2000).
Local radionecrosis can occur by reflux of the administered 90Y or 186Re colloid through the needle track or by not correct injection and has to be avoided by excellent puncture technique and immobilization of the treated joint. Such necrosis heals slowly (several months) and usually leaves only a small depigmented skin area. Kryotherapy or local ointment are mostly sufficient. Early surgical excision of the lesion should be avoided in favor of observing the process in patience. Kampen et al. (2006) recommend in the opposite early surgical excision or in 186Re-induced necroses hyperbaric oxygen therapy. In cases of necroses from 169Er, the thin wound will heal by conservative local treatment (Fig. 5a, b).
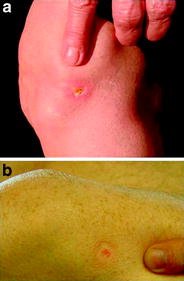

Fig. 5
Skin necrosis after Yttrium-90 RSO of the knee joint. a 6 weeks after RSO, b 4 months after RSO. After 5–6 months a small bright spot like vitiligo or scar after arthroscopy will remain. Caution: try to avoid (early) surgical manipulation!
Thrombosis: RSO might be ranked as low risk, due to immobilization, comparable to small or medium interventions with minor trauma according to surgical and perioperative classifications, provided that there are no individual risk factors. With respect to possible side effects, a general thromboembolic prophylaxis is not recommended in these patients. But prophylaxis with low-molecular-weight heparin is mandatory in cases with predisposing risk factors, of course (Fischer and Ritter 2006).
Joint infection is very rare observing strict sterile injection technique and may be achievable even in less than the “normal” rate of 1: 35,000 joint punctures (Kaiser and Kley 1992; Mödder 2008).
Cartilage damage was not observed even in the first known report about RSO (Ishido 1923) as well as in recent research (Isomäki et al. 1972; Pirich et al. 1999; Mäkelä et al. 2004). The fundamental reason is that the target of RSO-radiopharmaceuticals is the phagocytosing surface cells of the synovium, but cartilage has no phagocytotic capacity.
Risk of malignoma: Determination of the frequency of dicentric chromosomes in peripheral lymphocytes is the most sensitive method available today for detecting any radiation effects. In a recent study (Voth et al. 2006), the number of dicentric chromosomes was determined immediately before and 4 weeks after RSO with 90Y colloid. Prior to RSO, 26 dicentric chromosomes were found in 10,000 lymphocytes per individual (incidence rate 0.25 %); and after RSO, 34 were found in 10,000 cells (0.41 %). This difference was not statistically significant; thus, there is no evidence that RSO causes an increase in radiation effects, confirming the very low extent of whole-body radiation exposure engendered by 90Y- RSO.
In a meta-analysis of about 180 published studies and reports with more than 9,300 patients after RSO with 90Y only two cases (with chronic myelocytic leukemia after 4 years and lymphatic leukemia after 6 months) were found. The short intervening periods make RSO unlikely as a cause (Kos-Golja et al. 1997).
In 25 years of 90Y-RSO, no case of treatment-related malignancy has been established (Deckart et al. 1996). In a 7-year study of 1,228 hospital patients (143 received 90Y- RSO, 1,085 did not), it was found that those having received RSO had a lower rate of malignancies than those who got no RSO (Vuorela et al. 2003). In the author’s own experience with RSO since 1972—in the last decade with about 6,000 RSO per year—no one case of potentially RSO-induced malignancy was brought to his attention.
5 Radiopharmaceuticals
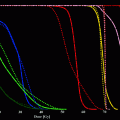
The choice of the radionuclide for RSO of certain joints is based upon the tissue penetration depth of the emitted radiation and upon the half-life of the radioisotope used. The penetration depth should be adequate to the thickness of the synovium in the joint to be treated. Too shallow penetration should be balanced against the potential hazard of too deep penetration. The half-life of the chosen radionuclide should be long enough to permit good distribution within the surface of synovium and effective adequate exposure. The half-life should be short enough to avoid excessive irradiation within the joint. And significant leakage should not happen.
The radiopharmaceuticals are β-emitters in sterile colloidal suspensions of an insoluble derivative (e.g., citrate for 90Y), with an appropriate particle size, thus prolonging retention time of radiopharmaceutical within the synovial capsule, longer than the decay time of the radioisotope.
The most common and approved radiopharmaceuticals used for RSO in Europe are
[90Y] yttrium-citrate ([90Y] colloid), only used for RSO of knee joints
[186Re] rhenium sulfide ([186Re] colloid), used for RSO of middle-sized joints
[169Er] erbium citrate ([169Er] colloid), used for RSO of small joints.
The physical characteristics of these radioisotopes are focused in Fig. 6.
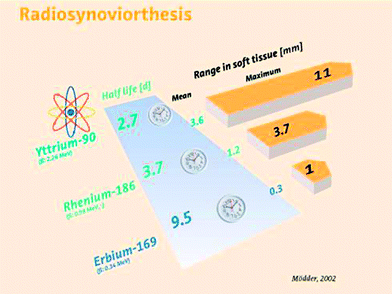

Fig. 6
Radioisotopes for radiosynoviorthesis
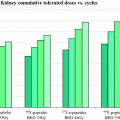
Recommended proven dosages are listed in Table 1.
Table 1
Established doses (MBq) of radionuclides for various joints (37 MBq = 1 mCi)
Joint | Yttrium-90 | Rhenium-186 | Erbium-169 |
|---|---|---|---|
Knee joint | 185–222 | ||
Glenohumeral joint | 74 | ||
Elbow joint | 74 | ||
Wrist joint | 55–74 | ||
Hip joint | 111–185 | ||
Ankle joint | 74 | ||
Talonavicular/subtalar joint | 55 | ||
Thumb base joint | 30 | ||
Metacarpophalangeal (MCP) joint | 22 | ||
Proximal Interphalangeal (PIP) joint | 18 | ||
Distal Interphalangeal (DIP) joint | 15 | ||
Cuneonavicular joint | 37 | ||
Tarsometatarsal joint | 22 | ||
Metatarsophalangeal (MTP) joint I | 30 | ||
Metatarsophalangeal (MTP) joint II–V | 22 |
Other radiopharmaceuticals, rarely used for RSO are Dysprosium-165-ferric-hydroxide and—with own practical experience in India—[166Ho] Holmium-166-hydroxy apatite, [153Sm] Sammarium-153-hydroxy apatite and [32P] Phosphorus-32 colloid. For developing countries, commercially available radiopharmaceuticals from far abroad are costly, and therefore other agents—produced locally in their own countries—may be of interest. A candidate for more spreading of RSO could become [32P] phosphorus colloid. This agent has a half-life of 14.3 days, maximum. β-energy 1.71 MeV, and tissue penetration depth 2.2 mm mean and 7.9 mm max. From these characteristics suitable for knee, RSO applied activity for knee joints should be 37–54 MBq (Liepe et al. 2011).
The advantage of using [188Re] rhenium is convenience, low cost, and onsite availability by using a 188W/188Re generator.
Due to the four decade–long clinical experiences, the applied activities for each isotope are well established, even regarding the complexity of the geometry of synovial joints. A formal dose finding study was performed by Voth et al. (2006), confirming in essence the established typically injected activities for Y-90, Re-186, and Er-169. For all three isotopes, the dose to the synovial surface of the injected joints amounts to about 130 Gy in case of the application of the recommended activities (Table 1). Theoretical and experimental evidence supported these results (Johnson et al. 1995).
Ideal radiopharmaceuticals for RSO should emit Beta energy sufficient to penetrate and ablate the synovial tissue, but not too intensive thus avoiding damage underlying articular cartilage or overlying skin and providing the smallest minimal lymphatic clearance. Radioisotopes have to be attached to particles, small enough to be phagocytozed and large enough to remain with uniform distribution within the joint cavity. They should be biodegradable. The ideal particle size should be about 10 nm (Ingrand 1973).
6 Mechanism of Action
“Synovitis is the villain of the drama” (Mannerfeldt), in rheumatic diseases causing brutal destruction of cartilage, bone, tendons, and ligaments correlated with pain, swelling, and loss of function. After intraarticular administration, the radioactive particles in colloidal form are taken up by phagocytosis in synovial macrophages lining the synovial cavity, with a homogenous distribution in the synovium 24 h later, demonstrated by autoradiography (Isomäki et al. 1972), partly delivered into the underlying tunica propria. A particle size of about 5–10 nm is essential to avoid leakage and provide homogenous distribution on the surface of synovium. Local β-radiation leads to coagulation necrosis, sclerosis, and fibrinoid necrosis of the synovial tissue including vessels and pain receptors, thus resulting in reducing effusion, swelling, and pain of the joint (Myers et al. 1989). Due to the fact that cartilage has no ability for phagocytosis, this tissue is no target for the radiation effects (Ishido 1923).
The remark “Synovitis is the villain of the drama” is not only valid for rheumatoid diseases but also for OA (activated arthrosis). Arthrosis with typical joint space narrowing as a result of cartilage defects is not associated with pain because cartilage has no nerves and vessels, as mentioned above. Only after detritus leads to synovitis, simple arthrosis escalates to inflammation (activated arthrosis = OA) with pain, swelling, and effusions (Otte 2000; Mödder 1995a, b, 2006). The rather good effects of RSO in OA (i.e., knee joint) will be missing if mechanical problems as severe instability and axe deviation are predominating (Fig. 4).
Simultaneous intraarticular injection of corticosteroids (i.e., triamcinolone hexacetonide or triamcinolone acetonide) is recommended because this might reduce local inflammation due to radionuclide instillation and prolong residence time of the radiopharmaceutical agent in the joint (Bridgman et al. 1971). An additional reason is the reduction of the often superposed layer of edema on the synovium so that the thin film of radioisotopes with limited penetration depth gets closer to the destructing pannus: resulting in improvement of the effect of RSO (Fig. 7).
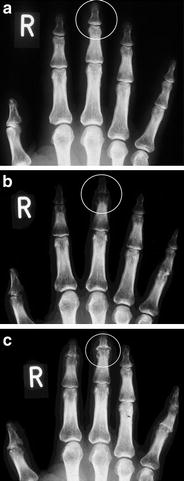

Fig. 7
X-rays of the fingers in a 40-year-old patient with rheumatoid arthritis (RA). Course-observation: a image 5/93, b 4/94: massive progression of the destruction of DIP III (despite gold and methotrexate), c 2/95 significant osseous repair of the DIP III after RSO 8/94 with [169Er] erbium
7 Patient Selection
Chronic inflammation of the synovial membrane is the most important aspect for selecting a joint for RSO, but under certain circumstances.
Rheumatic patients need systemic treatment with antirheumatoid drugs because rheumatism is a systemic disease. If after at least 6 months, few joints do not show adequate improvement even after corticosteroid injections into the affected joints these joints are selected for RSO, thus avoiding escalation of systemic therapy with its possible side effects. In monarthritis or oligoarthritis, RSO could be the therapy of first choice after failure of locally administered corticosteroids (Mödder 2001; Fischer and Mödder 2002).
Ideally, patients are referred to the radiotherapist for RSO by a rheumatologist or orthopedist with whom he closely cooperates. In this case, he may assume that careful specific diagnostic testing has taken place, that conservative therapy has been tried (and remained ineffective), and that adequate indication for RSO exists. In the case of a rheumatic disease, at least half a year of basic therapy should have been carried out. The guidelines of the German Society of Rheumatology require at least one failure of intraarticular corticosteroid before the joint will be regarded suitable for RSO.
Nevertheless, this does not relieves the radiotherapist performing RSO from the responsibility of verifying the adequacy of indication.
Orthopedic patients should be submitted after failure of local corticoid injection and/or ineffective conservative treatment. But also after a plenty of surgical interventions RSO might improve the complaints of the patient, i.e., after total knee replacement (Mödder and Mödder-Reese 2001) or effusions after arthroscopy. Some authors recommend RSO after arthroscopy as a routine to provide better results by increasing radicality in removing synovial swelling and still decreasing traumatisation (Kerschbaumer and Herresthal 1996; Thabe 1997) The time interval between arthroscopy or joint surgery (i.e., PVNS) and RSO should be planned for (4–) 6 weeks.
8 Diagnostic Studies Prior to RSO
Diagnostic studies prior to RSO basically include:
a careful medical history and clinical inspection with examination of joint properties and function provide basic information about the disease, its previous therapy, and the selection of the joints for RSO.
X-ray images, laboratory tests, and previous medical reports—usually brought along by the patient—might deliver more details.
a detailed patient instruction about the necessary preexaminations and the RSO itself is obvious. Then the patient confirms the informed consent by signature.
8.1 Ultrasound (Arthrosonography)
Ultrasound is an examination procedure whose importance cannot be overemphasized for the quality of the familiarity with the target area of RSO. Ultrasound evaluates synovial structure and thickness, extent of effusion, it assesses tenosynovitis or rotator cuff tears (shoulder), demonstrates osteophytes and capsule swelling (knee joint). Ultrasound is obligatory prior to performing RSO of knee joint to rule out problems in the case of a Baker`s cyst (Fig. 32). The sometimes difficult puncture of Baker’s cyst is simple if directed by sonography. By ultrasound can be controlled the decreased thickness of synovium after RSO, up to 30–40 % (Gratz et al. 1999).
A 7.5 MHz soundhead is necessary for routine studies.
8.2 Scintigraphy
“Bone scintigraphy” was predominantly used in the search for skeletal metastases. In the oncologic field, it has been quantitatively pushed back by tumor marker-screening in aftercare or by competing methods (CT scan, MRI). An adequate enlarged use of the method according to the rheumatologic and orthopedic field in combination with RSO may lead to an excellent replacement.
Multiphase scintigraphy with 99mTc-MDP (or similar radiopharmaceuticals) is the best diagnostic tool for detecting and demonstrating inflammation of the synovium, thus—taking findings of the clinical examination into account—selecting joints for RSO.
Radionuclide angiography
If immediately after injection images in quick sequences are made (sequence scintigraphy), the initial blood flow of the pictured region is demonstrated (1st phase).
Soft tissue scintigraphy:
In the 2nd phase (10 min p.i.), the blood pool is visualized thus detecting the degree of active inflammation of synovium (Fig. 8). In most cases, the actual joint candidates for RSO are checked in multiple views, e.g., feet in medial, lateral, and plantar view.


Fig. 8
The hand as “visiting-card of the rheumatoid patient” (soft tissue scintigraphy). Psoriatic arthritis with typical “stream-like” pattern
Joint soft tissue scintigraphy is the decisive examination for the detection of arthritis.
Bone scintigraphy:
The 3rd phase (after 3 h p.i.) assesses bone involvement in the painful process (bone scintigraphy). Combined with the 2nd phase, the study reveals nearly indispensable information in activated arthrosis (OA): A lesion in the 3rd phase without accumulation in the 2nd phase means: not activated arthrosis. In this case, no RSO is indicated. A focal accumulation in a joint in both phases means activated arthrosis (=OA). RSO should be successful. Similar view refers to other chronic joint diseases.
Skeletal scintigraphy reveals osseous alterations earlier than radiologic procedures. The X-ray method shows morphologic changes, whereas scintigraphy (as function-topography) already makes metabolic changes visible.
Somewhat more difficult is the prognostic estimation of success rate regarding the RSO, if an extended joint space narrowing or even a severe axial malposition exists. The scintigraphy can also prevent one from performing RSO in truly hopeless cases.
A study for evaluation of the effect of RSO on the early (soft tissue) and delayed (bone phase) uptake of 99mTc-biphosphonate and its relation to clinical outcome was performed in 41 knee joints affected by RA by Schwarz et al. (2003), prior to and 4 months after RSO. There was found a significant correlation between pre- and post-soft tissue scintigrams according to uptake and clinical outcome, but not in the delayed phase (bone scintigraphy). A similar result was described in a study concerning patients with PVNS (Kat et al. 2000). In our own experience also, bone scintigraphy may show an increasing good correlation if the study is performed later, about >12–24 months after RSO.
Whole–body scintigraphy:
For performing a scintigram of whole body simultaneously in anterior and posterior views, a special gamma camera is necessary. It gives an excellent overview over multiple joint involvement in (even seronegative) polyarthritis, demonstrating also the degree of focal rheumatic lesions (Mödder 1995a, b). In orthopedic patients, polyarthrosis is visualized (Fig. 9).
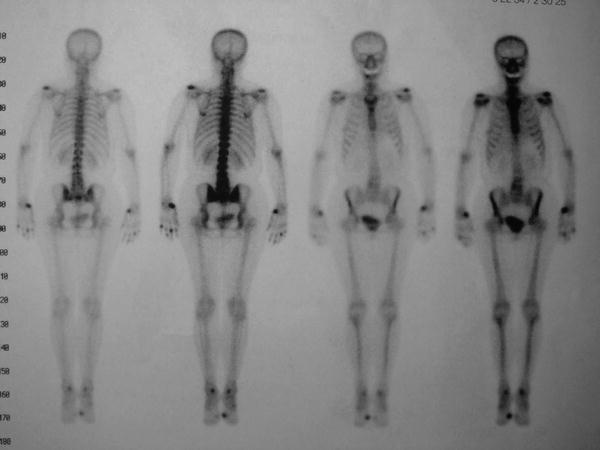

Fig. 9
Whole-body scintigram in a patient with polyarthrosis. The inflamed left acromioclavicular joint (AC) was treated with RSO after failure of three intraarticular corticosteroid injections
Single Photon Computerized Scintigraphy (SPECT):
SPECT gives a three-dimensional image of the joints. The method is recommended preferably in cases after total joint replacement.
MRI might be suitable for additional information in some patients (i.e., bone oedema, femur head necrosis).
9 Performance of RSO
9.1 Joint Puncture Technique
Joint punctures should be performed providing strict asepsis in a special room with high demands on cleanness. Because of radiation protection legislation, a room for RSO within the radiation control area is mandatory.
Joint punctures within the scope of RSO do not only serve for the intraarticular instillation of radiopharmaceuticals. During follow-up, e.g., it may sometimes be necessary to aspirate a joint effusion, to perform a sonographically directed puncture of a Baker’s cyst, or to perform another intraarticular corticoid injection.
Attention should be paid to a convenient position of the patient as well as for the one performing the puncture. The skin at the injection site needs to be disinfected. Excess local hair should be removed. Disposable instruments should be used. If possible, cover the area surrounding the joint with a sterile hole-sheet.
Knowledge of the topographic joint anatomy and mastery of the puncture technique is especially important for the procedure. Whereas a corticosteroid sometimes can be injected into the joint capsule, even with good results, a depot of a beta emitter out of the joint capsule could have disastrous consequences (extended necroses).
The radiation load for the physician is reduced if he first punctures the joint cavity with a nonactive syringe (containing local anesthetic) and then puts on the syringe with the radiopharmaceutical after a guaranteed perfect position of the needle. The utmost care is needed that the tip of the needle does not become displaced by this manipulation.
As a matter of principle: The nuclear therapist is alone responsible for the RSO (including the safe joint puncture).
Existing synovial fluid should be aspirated; however, a small amount of fluid should remain to allow better distribution of the radionuclide throughout the joint.
The injection needle and the puncture channel have to be flushed afterward (e.g., with the rest of local anesthetic), so that a radiation necrosis along the puncture channel can be avoided.
A good puncture technique is essential (Details see Mödder 1995a).
At every joint there are usually various puncture sites available. To acquire perfection thus avoiding an injury of the related structures (vessels, nerves, tendons, and tendon sheaths) it is advisable to use a preferred location, like a steady ritual. There exist good instructions for injection techniques, but some of the usual injection sites could be dangerous in case of RSO, e. g., hip joint (Details see special section and Mödder 1995a, b; Hatz 2009).
9.2 Fluoroscopy and Arthrography
For RSO of knee joints with 90Y arthrography is not recommended (and not necessary) because earlier studies had reported a dissolution of 90Y and colloid due to application of contrast medium containing EDTA as stabilizer. 90Y with a large size of colloidal particles of 10–20 nm has a high affinity to EDTA and other chelates. 169Er-erbium citrate is also bound to colloid particles of 10 nm (Bergmann and Höfer 1982). 186Re-rhenium sulfide is bound to smaller colloidal particles of 50–100 nm showing no affinity to EDTA (Saha 1992).
In an own in vitro study, we found that the disintegration of the radiocolloids observed in vitro in the presence of contrast-enhancing agents could be attributed to either the formation of EDTA complexes or radiolytic effects. Larger volumes (>0.06–1.0 ml) of contrast agents are necessary to mobilize the radionuclides from the colloids. As a consequence, the volume of the injected contrast agent should be as small as possible to confirm correct intraarticular contribution thus avoiding leakage (Schomäcker et al. 2005). We prefer iopromide (Ultravist®).
With exception of the knee all joints have to be punctured for RSO under fluoroscopic control, in most procedures completed by arthrography. Dye distribution predicts the distribution of radionuclide which is injected immediately afterward. By this control perfect needle position in the cavity of the joint is ensured.
Otherwise, the mandatory strict intraarticular injection of radiopharmaceuticals is not guaranteed. Best is a surgical picture intensifier (“C-arm”), for this allows a complete free access to all joints and the closest contact of the joint to the X-ray tube for optimal picture quality.
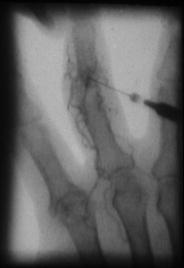
It must be strongly advised against punctures without fluoroscopic control. Even the most skilled and experienced in doing punctures (with surely few exceptions) would often be surprised, where he ended up with the needle, after the “blind” punctured pathological deformed joint becomes visible by a fluoroscopic image (Fig. 10).

Fig. 10
Arthrogram of a PIP-Joint. Intraarticular position of the needle, but not in the cavity. The contrast medium is injected into a villous with transportation by vessels. If this would happen with injection of Erbium-169 this would be followed by side effects
More examples for the importance of fluoroscopic control for absolutely exact injection technique are demonstrated in special Sect. 11.
9.3 Distribution Scintigraphy
RSO has to be finished by fixing the treated joint by a splint and as the last technical procedure a distribution scintigraphy has to be performed after RSO with 90Y and 186Re.
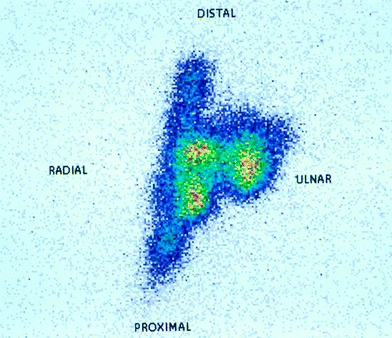
After RSO immobilization of the joint, it is essential to avoid leakage and necrosis of injection channel or skin caused by reflux and to avoid leakage of radioactive particles through the lymphatic vessels (Gratz et al. 1999). A splint is required for 48 h.
After removal of the splint 48 h later, the joint should be treated with care for 1 week, but then the patient should go into training of joint and muscles.
Distribution scintigram confirms the appropriate intraarticular distribution of the radiopharmaceutical. Scintigraphy is enabled after use of 90Y by its Röntgenbremsstrahlung, after use of 186Re by its gamma portion (140 keV, nearly like 99mTc) (Fig. 11).
A distribution scintigraphy is not possible with [169Er] erbium because of the short range (1 mm) of its beta particles.

Fig. 11
Distribution scintigram after RSO of the wrist dorsal. Through gentle tipping of the hand succeeds unproblematical a preferred distribution of the dye (and obviously consecutive also of the [186Re]) in the ulnar compartment. Note the corepresentation at a time of the tendon sheath of the extensor carpi ulnaris muscle
Figure 12 shows a synopsis of (a) soft tissue scintigram (99mTc), (b) arthrogram, and (c) distribution scintigram (186Re) as a proof for a perfect performance.
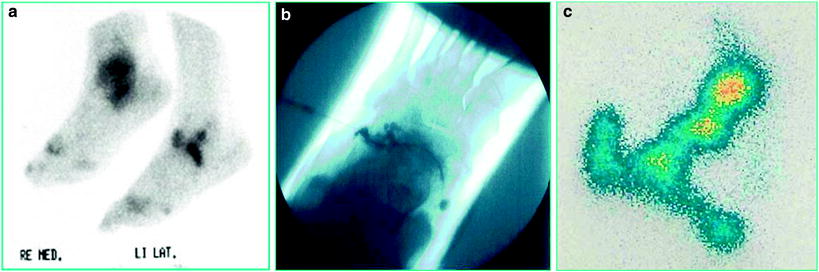

Fig. 12
The patient with RA should undergo a triple arthrodesis of the left foot. Is that a case for RSO? a Soft tissue scintigraphy detects inflammatory involvement in the talonavicular, subtalar, and calcaneocuboid joint. b Arthrogram after injection in the calcaneocuboid joint. c Distribution scintigram in the same pattern as scintigram demonstrating perfect distribution exactly in the identical joints marked in the diagnostic scintigram
10 Radiation Safety Considerations
For the patient: Very late radiation induced stochastic hazards have not been observed (Vuorela et al. 2003). In 186Re RSO, the effective dose to the whole body is estimated to be 30 times lower than in Iodine-131-therapy of benign thyroid diseases. For 169Er RSO, physical dosimetry gave an effective dose lower than 1 mSv/30 MBq (Manil et al. 2001).
Leakage of the radiopharmaceutical should be avoided by strict immobilization of the treated joints, which may be maintained without problems in outpatients. Then the leakage is very low, resulting in a whole-body exposure between 30 and 150 mGy (Klett 2006). A study of activity leakage and radiation exposure in RSO of the knee joint—measured by post therapeutic whole-body scintigraphy—showed that in 15 of 35 patients a leakage was verifiable. Three groups were examined: RSO 6–8 weeks after arthroscopic synovectomy, RSO alone as outpatient procedure, and RSO alone in hospitalized manner. There was no statistical difference between these modalities. Gonadal radiation dose could be neglected and also the morbidity rate for tumors because of a low body radiation dose (0.4 per 1,000). Conclusion: RSO can be used unlimited by patient age and independent of the therapeutic modality (Klett et al. 1999; Klett 2006).
For the personnel: 90Y produces the greatest part (90 %) of radiation exposure during RSO because of its beta energy of 2.1 MeV. Beta exposure hits the palmar surface of the left forefinger tip and thumb tip (right hander), with which the therapist fixes the injection needle. This beta radiation exposure for the fingers has to be controlled by wearing thermoluminescence β-finger-dosimeter (Fig. 13) during application of 90Y and preparation as well. Radiation protection has to be provided by use of: acrylic syringe protectors (Fig. 13), Nitril-, or Vinyl gloves, acrylic ring at the syringe cone or a forceps etc. (Brenner 2006; Liepe et al. 2003) (Fig. 14).
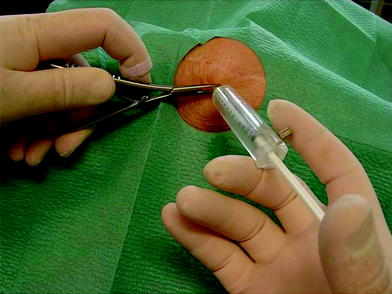
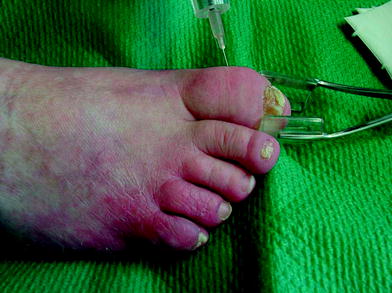

Fig. 13
Radioprotection—during RSO of a knee joint—for the therapist: Forceps lengthens the distance from the conus of the syringe when he has to change the syringe (Corticosteroid after injection of 90Yttrium). A beta radiation finger ring dosimeter measures the dose

Fig. 14
A forceps may be a useful tool to handle this psoriatic joint, simultaneously reducing fluoroscopic ionizing radiation upon the hand of the therapist
11 Special Section
Various joints are now systematically discussed. Essentials have already been presented in the previous part. This is now taken for granted and thus only few specific features need to be emphasized (details see Mödder 1995a).
11.1 Sternoclavicular Joint
The sternoclavicular joint may be involved in the case of psoriatic arthritis, especially in Sappho syndrome, but also in OA. This joint normally is completely or incompletely divided by a longitudinal disk.
11.2 Acromioclavicular Joint
In the region of shoulder two joints have to be distinguished—aside clinical examination—best possible with scintigraphy: acromioclavicular joint and glenohumeral joint (Figs. 15 and 16).
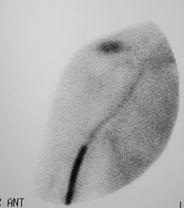
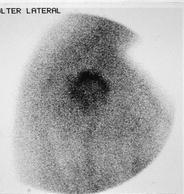

Fig. 15
Soft tissue scintigram shows inflammation of AC

Fig. 16
Soft tissue scintigram of the right glenohumeral joint (lateral view) demonstrates synovitis-like tracer accumulation in a patient with OA
11.3 Glenohumeral Joint
Apart from RA or OA also special cases may occur as “omarthritis of the elderly woman” or “Milwauckee shoulder” with very painful osseous destruction and bloody effusions.
Sonography:
Ventral vertical- and horizontal-cuts in the intertubercular sulcus show the long bicipital tendon, whose recess may also be filled in an effusion. One must always look for a partial or complete rotator cuff tears, usually with leakage of the effusion into the bursae (subacromial or subdeltoideal). However, rotator cuff tears is no contraindication of RSO because the intraarticular distributed radiopharmaceutical will not leave the glenohumeral cavity by leakage into periarticular tissue but enter the bursa subacromialis thus remaining in a closed cavity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree