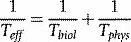Chapter 7 Therapy with unsealed radionuclides
Iodine-131 in the treatment of thyroid disease
Phosphorus-32 in the treatment of refractory myeloproliferative disease
Intra-articular and intracavitary treatments
Iodine-131 m-IBG therapy in neuroendocrine disease
Radiolabelled monoclonal antibodies in non-Hodgkin’s lymphoma
Radiation protection, waste and regulations
Introduction
where Teff, Tbiol and Tphys are the effective, biological and physical half-lives. The effective half-life is the important parameter when estimating radiation dose as it determines the duration and rate of delivery of radiation dose.
The subject is growing and this text has been limited to products with licences or marketing authorizations, but it should be noted that there are many interesting areas of development, such as the use of yttrium-90-labelled somatostatin analogues for neuroendocrine tumours [1–3].
Please note that where a product is unique to a particular company, the trade name has been given.
Iodine-131 in the treatment of thyroid disease
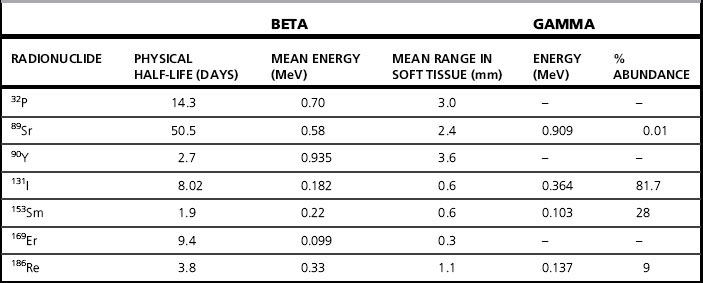
Iodine-131 (131I) is the radionuclide most widely used therapeutically. Iodine is readily concentrated in the thyroid gland and using 131I, emitting beta particles with a maximum range of 3 mm in tissue, allows a high radiation dose to be delivered to the thyroid and a low dose to the rest of the body. It is most commonly used in the form of sodium iodide[131I] for treatment of benign thyroid disease (thyrotoxicosis and non-toxic goiter) and in thyroid carcinoma. It has no role in medullary thyroid cancer. There are specific guidelines [4–6] from the Royal College of Physicians (RCP) in the UK, as well as from Europe and the USA for using radioiodine in the management of hyperthyroidism and of thyroid cancer. The characteristics of 131I are shown in Table 7.1. As specified by the European Association of Nuclear Medicine (EANM) and the Society of Nuclear Medicine (SNM) [5,6], it is essential that, before any treatment, all thyroid hormones, iodine-containing preparations and supplements and any other medications that could suppress thyroid uptake are discontinued for a sufficient length of time. Almost all thyroid treatments are given orally, as a capsule or as a liquid.
Thyrotoxicosis
In thyrotoxicosis, or hyperthyroidism, the thyroid gland is over-producing thyroid hormones. The possible approaches to radionuclide therapy have included giving sufficient radioiodine to render the patient hypothyroid and giving low activities of 131I in combination with anti-thyroid drugs. However, the RCP recommend that the aim of treatment should be to render the patient euthyroid, while accepting that there will be a moderate rate of hypothyroidism [4]. For a standard case of hyperthyroidism, the RCP suggest a guide activity of 400 to 550 MBq at first presentation. An alternative is to use pretreatment thyroid uptake measurements, with tracer activities of 131I, to calculate the activity to be administered to deliver a prescribed radiation dose. Such calculations require knowledge of the thyroid mass, the percentage uptake and the rate of clearance from the gland, requiring repeated measurements over a period of several days. Some centres may use measured uptake and thyroid mass but assume a standard turnover rate. There have been studies looking at the effectiveness of different treatment schedules and corresponding rates of hypothyroidism [7, 8]. Most treatments in the UK are administered to outpatients, although this will depend on the amount of activity prescribed, the patient’s home circumstances and national regulations.
Thyroid tumours
There is also a role for 131I in the treatment of well-differentiated thyroid cancer, when it is administered both for the ablation of thyroid remnant after surgery and for the treatment of metastases. Following total thyroidectomy, the aim of remnant ablation is to destroy any remaining normal thyroid tissue and any microscopic deposits of thyroid carcinoma [9]. The RCP guidelines [4] state that the usual activity administered for ablation is 3.7 GBq but that some centres may use a lower activity (1.1 GBq) and, as for thyrotoxicosis, some use a dosimetric assessment of uptake and clearance in order to prescribe an activity. By destroying any remaining thyroid tissue, the theory is that the only remaining source of thyroglobulin production is any remaining malignant cells, thus making the measurements of thyroglobulin level a sensitive test of any local recurrence or metastatic disease. Metastatic lesions have a lower avidity for iodine than normal thyroid tissue and it is customary to administer higher activities, for instance 7 GBq or more. Treatments for thyroid cancer require an in-patient stay, until the level of radioactivity has fallen sufficiently for safe discharge as outlined in the Medical and Dental Guidance Notes (MDGN) [10]. Gamma camera images, using the 364 keV photons of 131I, may be obtained after treatment to confirm uptake in residual thyroid, recurrence or metastases. Scanning protocols may also be used after surgery and before ablation. It may also be used for instance, to determine the completeness of ablation as part of a patient’s treatment. Iodine-123 may provide a suitable alternative, with better characteristics for imaging with a gamma camera. The first report of the use of 131I in treating metastatic thyroid cancer was in 1946 [11]. Even 50 years after that first report, much is being discussed and written about optimization of these treatments.
Phosphorus-32 in the treatment of refractory myeloproliferative disease
Phosphorus-32 (32P) is a pure beta emitting radionuclide, with a mean particle range in tissue of 3 mm and a maximum of 8 mm. It is available as a sterile solution of 32P orthophosphate in aqueous solution (sodium phosphate [32P]) which is administered either orally or as normally occurs, by intravenous injection. There is no requirement for an in-patient stay. The most common indication is the treatment of polycythaemia rubra vera (PRV), although the treatment may also be used in essential thrombocythaemia, a rare disorder. In the opinion of the EANM, the use of 32P for this indication is declining. However, there seems to be some agreement that it has a role in patients over 70 who are resistant to other treatments such as venesection and conventional chemotherapy [12].
Treatment regimens vary and are a matter for clinical judgment, with typical activities in the range 150–250 MBq. The EANM Guideline suggests two regimens in current use, based on using either an activity per surface area or a fixed starting activity which is incremented. The use of 32P to treat PRV was first reported in 1955 [13], but Parmentier [12], in a review in 2005 stated: ‘Few data are available regarding precise dosimetry in man’. An effective dose (ED) of 2.4 mSv/MBq is given by the International Commission for Radiation Protection (ICRP) in ICRP80 [14], with 11 mGy/MBq for both the bone surfaces and red marrow in ICRP53 [15]. Values of the same order of magnitude may be found in the literature.