Fig. 21.1
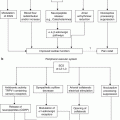
Autonomic nervous system schematic, with special emphasis on common anatomic pathways of the sympathetic nervous system (With permission © Mayo Clinic, 2010)
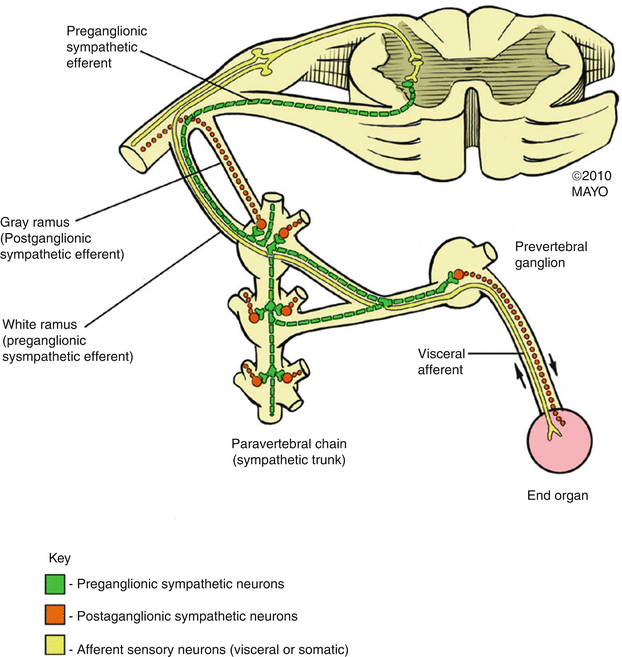
Fig. 21.2
Schematic of the central cord, exiting spinal nerve, and traditional anatomic path of the sympathetic nervous system that originates in the central lateral horn. Note that typical procedural targets include the lateral paravertebral chain (sympathetic trunk and ganglia) and/or the prevertebral ganglion/plexus (With permission © Mayo Clinic, 2010)
The sympathetic chain (paravertebral ganglia) extends from the top of the cervical spine down to the coccyx, traveling as two sympathetic trunks located along the anterolateral portion of the vertebral column. It is further subdivided into 23 sets of paired ganglia in the cervical [3], thoracic [12], lumbar [4], and sacral [4] regions, plus one single unpaired ganglion impar [2, 10–13]. Prevertebral ganglia, which provide neural intercession between the sympathetic chain and the postganglionic end-organ target, consist of specific ganglia and/or plexi located in the head, chest, abdomen, and pelvis. The major prevertebral sympathetic structures include the ciliary/otic/sphenopalatine/submaxillary ganglia, cardiac plexus, pulmonary plexus, celiac ganglia, superior and inferior mesenteric ganglia, and superior and inferior hypogastric plexuses [2, 10, 11].
The two anatomic areas most relevant to the discussion of this chapter are the cervicothoracic and lumbar ganglia regions. The cervicothoracic sympathetic region is principally composed of superior, middle, inferior, and stellate ganglia in the cervical region, along with 12 paired paravertebral ganglia in the thoracic region. The stellate ganglion, which is chiefly formed by the convalescence of inferior cervical and first thoracic ganglia elements, is of particular interest and lies ventrolaterally to the body of C7 with extension to the lateral portion of the T1 vertebral body [2, 11]. As mentioned previously, anatomic variants may include Kuntz Fibers which are postganglionic sympathetic branches from upper thoracic sympathetic ganglia (primarily T2 and T3) that may have direct neural connections to the brachial plexus external to the normal paravertebral (sympathetic chain) pathway.
Caudal to the stellate ganglion lies the thoracic sympathetic chain, which continues in a linear course along the dorsolateral aspect of the thoracic vertebral bodies; it is punctuated by paired segmental thoracic ganglia that lie just slightly caudad of the vertebral body midline (Fig. 21.3) [2, 9, 11]. The thoracic chain lies anterior to the head/neck of each rib in close approximation to the costovertebral interface and is bounded anterolaterally by the pleura of the lung [2, 11]. In the mid to lower thoracic regions, the sympathetic chain migrates to a more anterolateral position, relative to the vertebral bodies and lies at the anteromedial interface of the iliopsoas fascia as it further extends to the lumbar level. Typically, the lumbar sympathetic chain is found to have four discrete paired ganglia, but significant anatomic variation exists. Anatomic dissections have demonstrated a propensity for clustering of most significant lumbar sympathetic ganglia at L3, which tends to be the classical target of percutaneous-based pain interventions [2]. More specific discussions regarding thoracic and lumbar sympathetic anatomy will follow in the interventional technique sections.
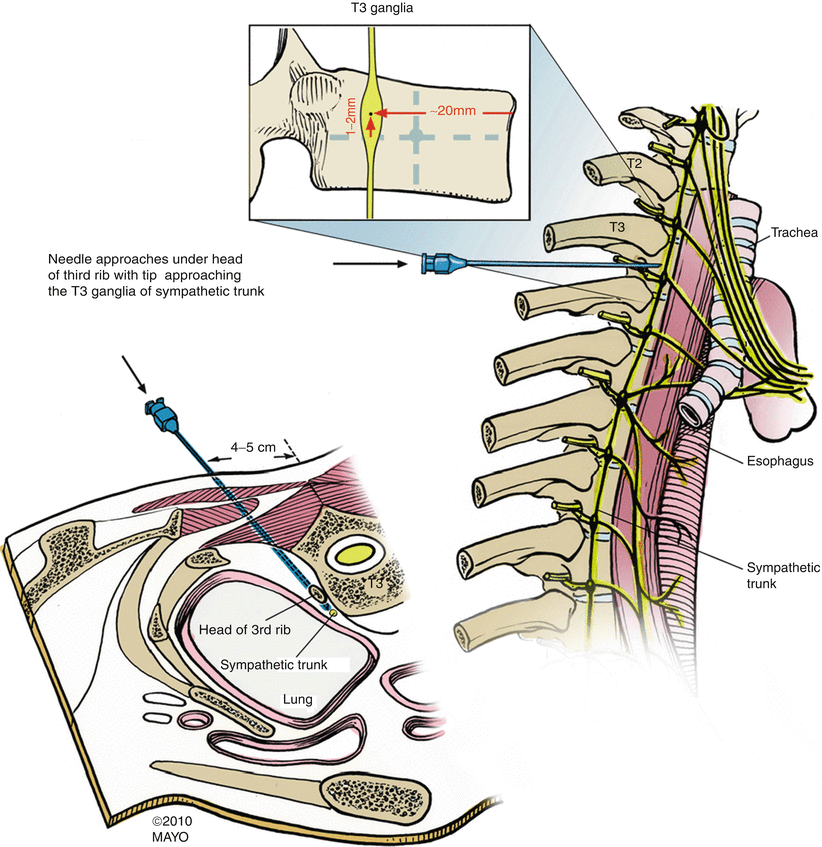

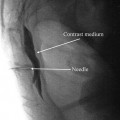
Fig. 21.3
Thoracic sympathetic ganglion block, using classic fluoroscopic technique, denoting the sagittal and axial views of the final position of the procedural needle tip (With permission © Mayo Clinic, 2010)
Methods of Neurolysis
Specific mention should be made of chemical neurolytic agents, since they are less commonly used and have unique properties that warrant particular attention. The most commonly used neurolytic chemicals are alcohol (33–100 %) and phenol (2–12 %, with or without glycerol additive). Alcohol is hypobaric relative to CSF, causes significant pain/burning upon injection, and induces Wallerian degeneration from the direct neurodestructive effects of ethanol. Notably, alcohol may allow for selective neurolysis of small sensory fibers (sparing motor) when used in low concentrations less than 33 % [14]. Phenol is hyperbaric relative to CSF when glycerol 4–10 % is added, has local anesthetic properties that minimize discomfort when injected, and may enable selective neurolysis of sensory nerves when used in small concentrations (typically 2–3 %). Phenol imparts neurolysis due to denaturing of protein, which may explain its predilection to cause vascular injury (risk of spinal infarction with subarachnoid use, erodes Dacron graft material, etc.). While less likely than phenol to cause direct vascular injury, alcohol has been associated with increased risk of vascular spasm and subsequent ischemic blood flow [14]. Lastly, phenol has been linked with arrhythmia and cardiovascular collapse; the mechanism is not fully elucidated, but likely relates to phenol’s sodium channel antagonist properties.
Continuous radiofrequency ablation (RFA) relies upon the application of heat, generated by continuous radiofrequency (RF) waves, to cause thermocoagulation of nerve fibers. This technology heats adjacent tissue to 80 °C, typically for 90 s. Recognize that sensory testing at 50 Hz and 1 V and motor testing at 2 Hz and 3 V may help establish whether intercostal/somatic nerves are being stimulated. If one is unable to elicit somatic nerve (intercostal) stimulation at 2 Hz and up to 1.5 V, it is much less likely that thoracic sympathetic ganglion RFA will cause injury to the segmental somatic nerve [8]. Additionally, post-RF neuritis sometimes develops after lesioning; this is usually self-limited, spontaneously resolves, and may be treated with steroid administration (prophylactically or after neuritis develops).
Thoracic Sympathetic Block
Specific Anatomy and Physiology
The thoracic sympathetic nerves typically consist of 12 paired paravertebral ganglia that punctuate the sympathetic chain at each thoracic vertebral level. These thoracic level paravertebral trunks travel dorsolaterally relative to the vertebral body, just anterior to the transverse process and posterior to the pleura of the lung [2, 11–13]. The superior most thoracic ganglion (T1) typically fuses with the inferior cervical ganglion (C8) to form the stellate ganglion in the majority of patients [2, 11–13]. The upper thoracic sympathetic chain runs anterior and just lateral to the head of the rib, with the ganglia located slightly caudad to the inferior edge of the head of the rib (Fig. 21.3). As one progresses down the thoracic spine, the sympathetic chain gradually moves closer to the anterolateral position on the vertebral body assumed in the lumbar region. The thoracic sympathetic ganglia lie just inferior of the true vertical midpoint of the vertebral body, though the anteroposterior position moves more ventrally as one descends down the thoracic spine [8, 9]. To be sure, once at the T11 level, the low thoracic sympathetic chain has assumed a much more anterolateral position with individual ganglia located against the lateral surface of the vertebral body.
As mentioned previously, anatomic variants may include Kuntz Fibers, which are postganglionic sympathetic branches from the T2 and T3 (possibly T4) sympathetic ganglia that may have direct neural connections to the brachial plexus external to the normal paravertebral (sympathetic chain) pathway. The clinical relevance of this common anatomic pathway (10–20 % prevalence) is that a perfectly performed stellate ganglion block may not result in a complete sympathectomy for the entire upper extremity. Kuntz fibers, arising from the upper thoracic sympathetic ganglia, may provide unrecognized sympathetic innervation to the upper extremity [3, 6]. Consequently, it is possible to perform a successful stellate ganglion block that causes only a partial sympathectomy to the upper extremity due to unblocked nerves of Kuntz; this may appear clinically indistinguishable, in terms of asymmetric extremity temperature change, from a successful stellate blockade in patients without significant Kuntz Fibers. Thus, one must consider that sparing of Kuntz Fibers may be responsible for a stellate ganglion blocks that fail to relieve pain (regardless of evidence for successful sympathectomy). The primary clinical impetus for development of thoracic sympathetic blocks was an attempt to target these Kuntz Fibers at T2 and T3, though the techniques described can readily be applied throughout the thoracic spine.
Indications and Contraindications
Indications for thoracic level sympathetic block include hyperhidrosis, upper extremity vascular malperfusion (Raynaud’s), and upper extremity or thoracic level sympathetically maintained neuropathic pain, along with visceral pain syndromes from the heart, lung, and/or esophagus. In general, because of the overlap in anatomic territory and existence of Kuntz fibers, one should consider upper thoracic level (T2 or T3) sympathetic blockade for any of the commonly accepted indications described for the stellate ganglion. Along with thoracic and upper abdominal visceral pain, consideration should be given for post-thoracotomy pain (particularly if sympathetic features are evident), postherpetic neuralgia, frostbite injuries to the upper extremity, and phantom breast pain [13].
Contraindications include the typical neuraxial absolutes of localized infection (skin or adjacent structures), systemic infection, and bleeding diathesis or coagulopathy. Relative contraindications relate primarily to the underlying function of adjacent anatomic structures, namely, pulmonary impairment and/or aneurysm of the great vessels (aorta or vena cava).
Complications and Expected Side Effects
Expected side effects from thoracic sympathectomy include ipsilateral Horner’s syndrome, adjacent somatic nerve block, and cardiac accelerator fiber block. Unexpected, though entirely possible, complications include intrathecal, subdural, epidural, intravascular (intercostal, azygous, aorta) injections. Also possible, though very unlikely, is the danger of esophageal perforation if the needle is placed too anteriorly. The most feared of the complication is unintended puncture of the lung pleura and consequent development of pneumothorax. Pneumothorax may present with a delayed clinical presentation, which necessitates informing the patient of probable warning signs and when to seek medical attention. If using neurolytic techniques, damage to somatic nerves may occur from spread through the epidural, paravertebral, intervertebral foramen, or even intrathecal space (less likely); this may result in significant sensory and/or motor deficits that are not reversible.
Procedural Technique for Block Neurolysis
There are various descriptions of blocks and/or neurolysis to the thoracic sympathetic chain, but our discussion will focus on percutaneous approaches and intentionally omit reviews on surgically open and endoscopic techniques. One should also assume that real-time imaging, typically with ultrasound and/or fluoroscopy, should be utilized in order to optimize the safety, accuracy, and precision of these techniques. In particular, without the ability to visualize the lung, the risk of pneumothorax cannot be entirely eliminated. The technical procedure is similar throughout the thoracic spine, but the details described below are most specific to the T2–3 levels [7, 8, 11, 13]:
Get Clinical Tree app for offline access
1.
Plan to use a posterior approach, placing the patient prone.
2.
If using fluoroscopy, orient the image intensifier in the true AP position, slightly oblique (15–20°, ipsilateral), with enough cephalad angulation to be in-plane with the neck of the rib at T2 or T3. This will allow coaxial placement of the procedural needle to a target point just anteroinferior to the head of the T2 or T3 rib (where the thoracic ganglion lies, see Fig. 21.3).
3.
Initially, enter the skin approximately 2.5–5 cm lateral from true midline (depending upon degree of obliquity used) and coaxially advanced to the inferior edge of the rib or transverse process as applicable, depending upon degree of cephalad angulation used.
4.
After touching bone, minimally redirect inferiorly until passing through the costotransverse ligament. The costotransverse ligament can be “felt” by a distinctive pop, or a loss or resistance technique can be utilized with a fluid or air-filled syringe. In either case, passage through this ligament heralds arrival at the retropleural space.
5.
The needle should be closely approximated to the lateral edge of the adjacent vertebral body. The final needle tip position will be approximately just cephalad and posterior to the true midpoint along the dorsolateral aspect of the T2 or T3 vertebral body (Fig. 21.3).
6.
Injection of contrast will demonstrate spread along the dorsolateral aspect of the thoracic vertebral column. If one breaches the parietal pleural, the lung dome will be outlined and the needle is too lateral (monitor patient for pneumothorax).
7.
Proper sterile technique should be observed at all times, with appropriate monitoring established beforehand and local anesthetic infiltration taking place prior to the placement of the procedural needle. Aspiration should be negative for CSF, blood, and air before injecting local anesthetic, chemical neurolytic agents, or applying radiofrequency ablation. Care should be taken to verify no foraminal spread of contrast prior to neurolysis. Lastly, the use of local anesthetic test doses and/or digital subtraction angiography will help elucidate unintended vascular uptake prior to neurolytic procedures.
8.
Ultrasound procedural pearls




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree