Chapter 17 The term “thyroid” is derived from the Greek word for “shield” because of the gland’s shape and relationship to the laryngeal thyroid cartilage. The thyroid gland has a dual embryonic origin.1–3 The two thyroid cell types, thyroid follicular cells (thyrocytes) and parafollicular (C-cells), are derived from all three germ cell layers.3 The most abundant cells, the follicular cells, arise from the thyroid anlage. The development of the thyroid gland begins as a bud of epithelial proliferation in the floor of the primitive pharynx between the developing tuberculum impar and copula of the tongue anlage, around 24 days’ gestation.4 This thyroid anlage soon forms a ventral outgrowth known as the thyroid diverticulum. The progenitor follicular cells proliferate distally and then laterally, leading to the characteristic bilobed appearance of the gland connected by an isthmus. As the embryo grows, the developing thyroid gland descends anterior to the hyoid bone and larynx, forming the thyroglossal duct. Because of the close association of the developing thyroid gland and embryonic heart, it is thought that the descent of the heart results in the thyroid gland being pulled.3,5 The thyroid gland remains connected to the tongue by the thyroglossal duct. At approximately 7 weeks’ gestation, the thyroid gland reaches its final site in front of the trachea and the thyroglossal duct disappears.4 The original opening of the thyroglossal duct persists as a vestigial pit at the base of the tongue called the foramen cecum.6,7 About 15% to 75% of people have a pyramidal lobe, which is derived from the lower part of the thyroglossal duct and extends upward from the isthmus.8 Around the time the thyroid gland reaches its final position, it merges with the two lateral anlagen or ultimobranchial bodies, resulting in the incorporation of the C-cells (parafollicular cells) into the thyroid gland. The ultimobranchial bodies are a pair of transient embryonic structures derived from the endoderm of the fourth pharyngeal pouch and the ectoderm of the fifth pharyngeal pouch, into which the C-cell precursors migrate from the neural crest.9,10 The thyroid follicular cells continue to organize the thyroid follicles. As the ultimobranchial bodies merge with the thyroid, their C-cells disperse within the interfollicular space.4 Remnants of the ultimobranchial bodies, or solid cell nests, are seen postnatally and are usually located in the middle third of the thyroid lateral lobes.11 The hypothalamus synthesizes and secretes thyrotropin-releasing hormone (TRH), which is carried to the pituitary gland by the hypothalamic-pituitary portal venous system.12–14 Once in the pituitary gland, TRH stimulates the synthesis and secretion of thyrotropin (thyroid-stimulating hormone [TSH]) from the anterior pituitary gland. TSH binds to receptors in the thyroid gland, stimulating follicular cell production and secretion of T4 and T3. Thyroid secretion and serum concentrations of T4 and T3 are maintained by a negative feedback loop involving inhibition of TSH and TRH secretion by T4 and T3.15,16 Iodide is actively transported into the follicular cells by the sodium-iodide symporter at the basolateral membrane.17–21 Thyroid peroxidase (TPO) oxidizes iodide into its chemically active form. Thyroglobulin in the follicular lumen serves as a matrix for the synthesis of T4 and T3. First, TPO catalyzes the iodination of selected tyrosyl residues in thyroglobulin in a process known as iodination and organification. This results in the formation of monoiodotyrosine (MIT) and diiodotyrosine (DIT). TPO then catalyzes a coupling reaction in which two iodotyrosines are coupled to form T4 or T3. Iodinated thyroglobulin is stored as colloid in the follicular lumen. When needed, thyroglobulin is internalized into the follicular cell and digested in lysosomes. Subsequently, T4 (80%) and T3 (20%) are released into the bloodstream. MIT and DIT are deiodinated and released iodide is recycled for hormone synthesis.3 C-cells produce thyrocalcitonin, which is important in calcium homeostasis. The thyroid gland is highly vascular being supplied by paired superior thyroidal arteries (first anterior branches of the external carotid arteries) and inferior thyroidal arteries (branches of the thyrocervical trunks that originate from the subclavian arteries). The thyroidea ima is an inconstant single vessel that has a variable origin but usually arises directly from the aortic arch or innominate artery and helps supply the inferior thyroid gland. Venous drainage is via the superior and middle thyroid veins, which drain into the internal jugular veins, and the inferior thyroid veins, which often join to form a single trunk draining to the left brachiocephalic vein. Lymphatic drainage is extensive and multidirectional. The thyroid gland is innervated by the vagus nerve and the cervical sympathetic neural plexus.22 Ultrasonography is usually the first choice of imaging in pediatrics because it is noninvasive, is readily available, and does not utilize radiation. A normal thyroid gland will have homogeneous echotexture, which is slightly hyperechoic relative to adjacent neck muscles.23,24 Colloid follicles are commonly seen as small (less than 3 millimeters [mm] in diameter) anechoic cystic areas. Occasionally, the follicles contain inspissated colloid, which appear as punctate echogenic foci (Fig. 17-1).25 Figure 17-1 Normal thyroid ultrasound. Nuclear scintigraphy provides morphologic and functional information about the thyroid gland. Thyroid scintigraphy is performed using intravenous Tc-99m pertechnetate (99mTcO4) or oral Na I-123 (I-123) (Table 17-1). Because of the large radiation dose to the thyroid gland (approximately 0.01 to 0.03 gray [Gy] per microCurie [uCi] administered), I-131 is not used for routine diagnostic imaging.26,27 The normal thyroid gland shows homogeneous radiopharmaceutical uptake and distribution in both lobes. The isthmus of the thyroid gland often demonstrates slightly less activity than the right and left thyroid lobes. Normal I-123 24-hour uptake ranges from 10% to 30%. The normal thyroid gland (because of its iodide content) has a density of approximately 80 to 100 Hounsfield units on CT. A well-visualized gland usually indicates a normally functioning thyroid, whereas a poorly seen gland correlates with poor thyroid function. The injection of iodinated contrast material diffusely and homogeneously enhances the gland.22 The use of iodinated contrast agents will alter radioactive iodine uptake, whereas gadolinium contrast material will not. The normal thyroid gland shows homogeneous signal intensity slightly greater than muscle on T1-weighted images. On T2-weighted images, the thyroid gland is relatively hyperintense to muscle. Following contrast administration, the gland enhances diffusely and homogeneously. Hypothyroidism is the most common disturbance of thyroid function in children. It may be congenital (Box 17-1) or may be acquired in childhood or adolescence (Box 17-2). The thyroid gland produces hormones that play a vital role in regulating many cellular and physiologic activities. Untreated congenital hypothyroidism in early infancy results in profound retardation of growth and neurocognitive development (cretinism). Untreated hypothyroidism in older children leads to growth failure as well as slowed metabolism and impaired memory. Hypothyroidism in the newborn may be permanent or transient. Congenital hypothyroidism with lower than normal T4 causes retardation of growth and neurocognitive development if left untreated. The incidence of congenital hypothyroidism in the United States has dramatically increased over the last two decades, from 2.9 cases per 10,000 births in 1991 to nearly 4 cases per 10,000 births in 2000.28,29 All states now require all newborns to be screened for hypothyroidism. Etiologies, Pathophysiology, and Clinical Presentation: The majority of cases of congenital hypothyroidism are caused by thyroid gland dysgenesis (see Box 17-1). Thyroid dysgenesis refers to a developmental defect of thyroid morphogenesis. The three types are (1) ectopia, (2) aplasia (athyrosis), and (3) hypoplasia. Imaging: Imaging is not routinely used to diagnose congenital hypothyroidism. According to the most recent recommendations for congenital hypothyroidism in newborns by the American Academy of Pediatrics, the American Thyroid Association, and the Lawson Wilkins Pediatric Endocrine Society, thyroid imaging in congenital hypothyroidism is optional because of controversy regarding the risk-benefit ratio and uncertainty whether imaging findings have any bearing on patient management.30 Diagnostic studies for congenital hypothyroidism can include ultrasonography and thyroid scintigraphy with 1-123 or 99mTcO4. Use of both ultrasonography and thyroid scintigraphy has been shown to provide a more complete depiction of congenital hypothyroidism in the newborn than either study performed alone.31 99mTcO4 or I-123 can be used to help determine if thyroid dysgenesis is the cause of hypothyroidism (see Table 17-1). In patients with thyroid agenesis, the test fails to demonstrate functional thyroid tissue. It is important that the images include the oropharynx and upper neck as well as the upper portion of the chest so that an ectopic thyroid gland can be excluded. 99mTcO4 scintigraphy demonstrates a round or oval area of uptake in the midline of the upper neck in most cases of ectopia (Fig. 17-2). The ectopic gland may occupy a lingual (most common), sublingual, or prelaryngeal location. Mediastinal and lateral locations are rare. Functional thyroid tissue may be identified in more than one location, most commonly in the lingual and sublingual regions. It is unusual to identify thyroid tissue in its normal location in the presence of an ectopic gland. Patients with an ectopic thyroid gland will usually have hypothyroidism. In some unusual cases, the ectopic gland is capable of secreting sufficient thyroid hormone such that hypothyroidism is not apparent on neonatal screening. These patients often present with signs of ectopia later in life when the hyperstimulated gland enlarges and causes local symptoms. Figure 17-2 Normal and abnormal thyroid scans. In cases of dyshormonogenesis, 99mTcO4 scintigraphy will demonstrate a normally positioned thyroid gland that may or may not be enlarged (e-Fig. 17-3). If dyshormonogenesis is suspected, a perchlorate washout test may be performed. Perchlorate is actively transported into the thyroid gland with a greater affinity than iodide and is, therefore, a competitive inhibitor of the thyroid iodide trap. During unimpaired thyroid hormonogenesis, iodide entering the thyroid gland is rapidly oxidized and iodinates tyrosine, forming MIT and DIT, with subsequent coupling of MIT and DIT to generate T4 and T3. Intrathyroid deiodination of the iodinated tyrosines and thyronines results in a very small pool of thyroidal inorganic iodide. Any congenital or acquired condition associated with a defect in iodide organification may yield a higher intrathyroidal inorganic iodide concentration. The perchlorate discharge or washout test is a means of estimating the size of this intrathyroidal “free” iodide pool, thereby detecting and roughly quantifying disturbances in iodide organification. The perchlorate discharge or washout test is performed by giving the patient an oral dose of I-123, followed by a dose of perchlorate and measuring the “washout” (see Table 17-1). The perchlorate test will be negative in patients who do not have an organification defect and also when enzymatic defects are present in the synthetic pathway beyond the point of organification.32–34 e-Figure 17-3 Abnormal thyroid scans. Chronic Autoimmune (Hashimoto) Thyroiditis: Acquired hypothyroidism is caused by many factors in the pediatric population (see Box 17-1). Chronic autoimmune (Hashimoto) thyroiditis is the most common cause of acquired hypothyroidism in children and adolescents in iodine sufficient areas. It is more common in girls than in boys and increases in frequency with age during childhood and adolescence.35–37 Etiologies, Pathophysiology, and Clinical Presentation: Chronic autoimmune thyroiditis is a complex, thyroid-specific T-cell mediated disease with a strong genetic component.38–40 It often coexists with other autoimmune diseases and may also be expressed as part of an autoimmune polyendocrine syndrome type 2.41,42 The two major forms of the disorder are goitrous autoimmune thyroiditis and atrophic autoimmune thyroiditis with the common pathologic feature being lymphocytic infiltration and the common serologic feature being the presence of high serum concentrations of antibodies to TPO and thyroglobulin. Approximately 2% of all surveyed adolescents have serum TSH levels indicating hypothyroidism.43 The most common physical finding at presentation is a goiter, along with growth retardation and short stature.35 The growth delay is usually insidious in onset and may be present for several years before other symptoms occur.44 Other common symptoms include changes in school performance, sluggishness, lethargy, cold intolerance, constipation, dry skin, brittle hair, facial puffiness, and muscle aches. If the cause is from hypothalamic or pituitary disease, the patients may have headaches, visual symptoms, or pituitary disease manifestations. Imaging: Most physicians consider the presence of serum antithyroid antibodies as sufficient evidence for chronic autoimmune thyroiditis, and thyroid ultrasonography or radionuclide scanning are rarely indicated. Children with central hypothyroidism should undergo cranial imaging, preferably MR (with contrast), and tests for other pituitary hormone deficiencies. Ultrasound findings are nonspecific but include an enlarged relatively hypoechoic gland with coarse heterogeneous echotexture. Less commonly, the echogenicity of the gland is increased relative to adjacent muscle. Fibrotic septations in the chronic form may produce a pseudolobulated appearance of the parenchyma. Multiple discrete, hypoechoic, 1 to 6 mm micronodules may also be seen (Fig. 17-4, A to C). Figure 17-4 Chronic autoimmune (Hashimoto) thyroiditis. In the early (preclinical) stage of Hashimoto thyroiditis, elevated I-123 uptake values with diffusely increased radionuclide activity may be seen. This happens because the initial mild decline in circulating thyroid hormone causes a compensatory rise in TSH secretion that stimulates the gland. Thyroid follicles may demonstrate a variable response to the chronic TSH stimulation, leading to patchy follicular proliferation. On the thyroid scan, this phenomenon manifests as patchy areas of increased activity (follicles that respond to TSH) and of decreased activity (those that do not respond). As more thyroid parenchyma is replaced by fibrous tissue, the radionuclide uptake becomes nonuniformly decreased (see Fig. 17-4, D).45
Thyroid and Parathyroid
Thyroid Gland
Physiology
Anatomy
Normal Findings
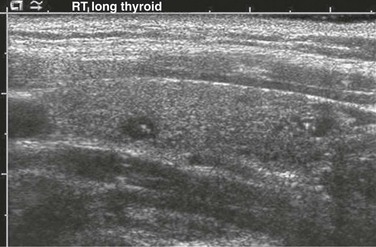
Small anechoic foci with a central hyperechoic focus represent colloid follicles, which are a normal finding.
Hypothyroidism
Congenital Hypothyroidism
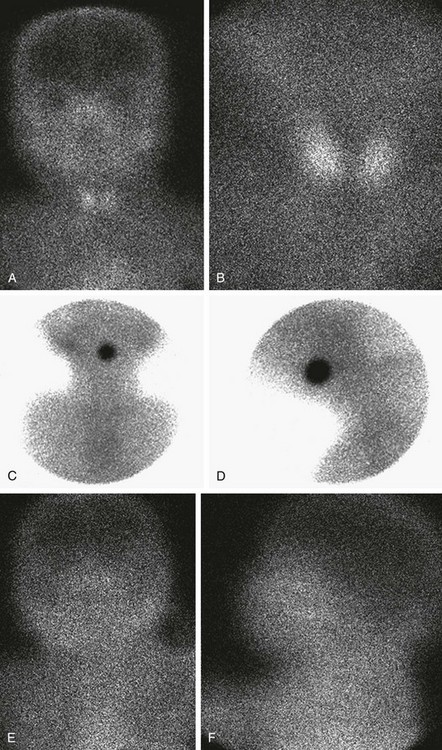
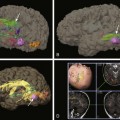
A and B, Normal thyroid anatomy. Anterior and close-up (pinhole) views show normal bilobate architecture and normal position. Thyroid function cannot be meaningfully estimated from these images in an infant with known thyroid insufficiency. C and D, Thyroid ectopia. Anterior and lateral views of the neck show a solitary rounded focus of radiopharmaceutical trapping at the base of the tongue. No clinical difference exists between the terms lingual and sublingual, and they are used interchangeably. E and F, No thyroid is identified. Anterior and lateral views show no functioning thyroid tissue. Although this finding suggests thyroid agenesis, it is nonspecific because severely decreased thyroid function, particularly resulting from maternal thyrotropin receptor–blocking antibody, also may have this appearance.
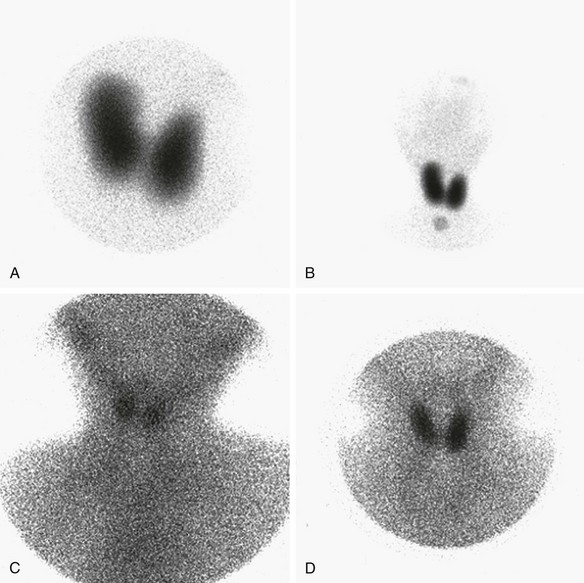
A and B, Organification defect. A, Anterior pinhole view. A bilobed thyroid is present in the neck. It shows diffusely increased uptake. B, Anterior prominently sized thyroid compared with the neck and the head. C and D, Anterior pinhole views of poorly functioning thyroid from two patients. A bilobed thyroid is normally located within the neck. Trapping by the thyroid is poor with high body background. The thyroid may appear small (C) or normal (D). This pattern can be seen in patients with thyroid gland hypoplasia or a transient insult, such as maternal antibodies. Scintigraphically, these entities cannot be distinguished.
Hypothyroidism in Children and Adolescents
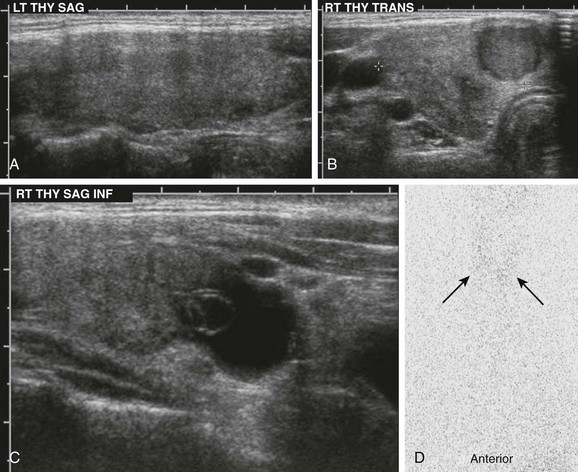
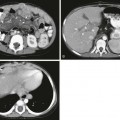
A, Ultrasonography demonstrates an enlarged gland that is relatively hypoechoic with heterogeneous echotexture. B and C, The right lobe of the same patient has a hypoechoic nodule and cystic changes. D, The I-123 scan shows diffuse decreased uptake (arrows). The 24-hour uptake was only 0.5%.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree