Julius Chapiro • Rafael Duran • Jean-François Geschwind
Intrahepatic cholangiocarcinoma (IHC) is the second most common primary hepatic neoplasm after hepatocellular carcinoma.1 With an annual incidence of 5,000 new cases in the United States and similar rates in Europe, it remains a rare cancer, although rates are considerably higher in Japan and other Asian countries.2 At the time of diagnosis, patients with IHC usually present with advanced stage disease, thus only 30% of the patients are candidates for surgical treatment.3 The overall prognosis in this disease is dismal, and not even surgical candidates achieve survival rates much higher than 30% at 5 years after resection with curative intent.4 Systemic chemotherapy has been disappointing regarding survival benefits for patients, and most regimens resulted in a median survival of no more than 12 months.5 Most regimen include gemcitabine and various combinations of 5-fluorouracil with cisplatin. However, until today, no randomized phase III trial exists to evaluate different chemotherapy regimen in IHC, and there has been great interest in exploring other modalities of treatment. In particular, multiple catheter-based intra-arterial therapies are possible to effectively treat patients with unresectable IHC. Unlike surgical or ablative approaches, intra-arterial techniques are not limited by tumor size, number of lesions, or proximity of major sensitive structures. Although mostly hypovascular, IHC still derives its blood supply from hepatic arteries, making intra-arterial delivery of anticancer agents (chemotherapeutic drugs or radiation) a potentially attractive therapy. The following describes the most commonly used intra-arterial therapies for IHC and discusses the available clinical evidence.
CONVENTIONAL TRANSARTERIAL CHEMOEMBOLIZATION
Background
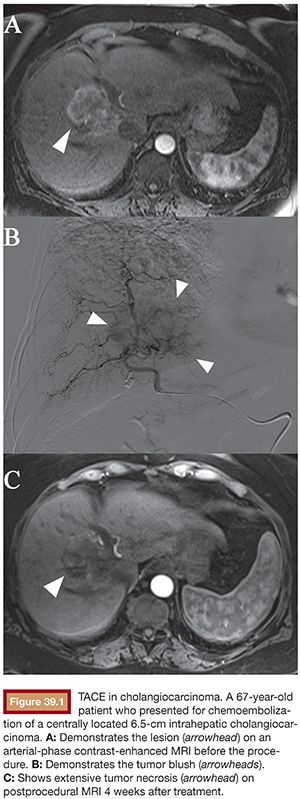
Of all the intra-arterial therapies used in the treatment of unresectable IHC, conventional transarterial chemoembolization (cTACE) is the most commonly used (Fig. 39.1). During this procedure, a mixture of chemotherapeutic agents combined with an oil-based contrast medium (Lipiodol or Ethiodol) is selectively delivered to the tumor-feeding artery. Lipiodol has unique properties and functions as an embolizing agent as well as an emulsion carrier for chemotherapeutic drugs.6 Doxorubicin is the most widely used single chemotherapeutic agent worldwide, yet the mix of doxorubicin, cisplatin, and mitomycin is the preferred combination in the United States and Europe.7 After injection of the drug-containing emulsion, embolic materials (such as gelfoam, polyvinyl alcohol [PVA] particles, or trisacryl gelatin [TG] microspheres) are administered with the purpose to cause stasis in segmental and subsegmental arteries, thus preventing washout of the previously deposited material.8
Clinical Evidence
One of the very first prospective trials designed to evaluate feasibility, safety, and overall survival after cTACE enrolled 17 patients with pathologically proven, mass-forming unresectable IHC. Eleven patients (65%) received cTACE as the first-line therapy, whereas 6 patients (35%) had received previous treatment including systemic chemotherapy and radiation therapy. On this protocol, chemotherapeutic agents used for most patients were 100 mg cisplatin, 50 mg doxorubicin hydrochloride, and 10 mg mitomycin-C. Most patients (82%) tolerated the procedure well and no life-threatening events were reported. In terms of efficacy, two of the patients were bridged to successful surgical resection after cTACE, and the median overall survival (OS) was 23 months.9 Another more recent single-center study from Germany retrospectively analyzed a larger cohort of 115 IHC patients treated with cTACE and confirmed the previously described results. Patients were repeatedly treated with cTACE (mean of 7.1 sessions per patient), yielding good tumor response rates (57.4% with Stable Disease [SD]). After a total of 819 TACE procedures, the overall safety profile and tolerability was exceptionally good for the entire cohort. However, it must be noted that no homogeneous treatment protocol was applied and various drugs and their combinations (mitomycin-C, gemcitabine, and cisplatin) were injected. Finally, the mean OS was 20.8 months with a 3-year survival of 10%, which is in agreement with the previously reported results.10 Another important two-center experience demonstrated the synergistic potential of cTACE with systemic chemotherapy. Here, a total of 62 patients with unresectable IHC (37 of which were pathologically proven) underwent repetitive cTACE treatment, and the used drug combination consisted of mitomycin-C, doxorubicin, and cisplatin. With only minor systemic toxicities and no disease-specific mortality 30 days after the initial treatment, cTACE was safe. Interestingly, the most important finding of this study was the significantly higher median survival rate in patients who received systemic chemotherapy when compared with those who did not receive any (28 months vs. 16 months, respectively). Furthermore, cTACE can also play an important role as an alternative adjuvant therapy for patients who underwent radical surgery. To explore this setting, a retrospective analysis included 125 IHC patients who underwent radical surgery with curative intent. A total of 53 patients (42%) received cTACE with various drug combinations (fluorouracil, carboplatin, epirubicin and hydroxycamptothecin, gemcitabine). A comparison of this group of patients with 72 nontreated individuals revealed no significant difference in the tumor recurrence rate. However, patients treated with TACE showed some survival benefits as compared to the nontreated group (1-, 3-, and 5-year OS of 69.8%, 37.7%, and 28.3% and 54.2%, 25.0%, and 20.8%, respectively). Of note, the results of this retrospective study must be interpreted with great caution primarily because of a highly inhomogeneous treatment protocol.11
TRANSARTERIAL CHEMOEMBOLIZATION WITH DRUG-ELUTING BEADS
Background
The advent of drug-eluting beads (DEBs) provided an additional therapeutic option for patients with unresectable IHC. Instead of the oily medium, DEB-TACE delivers polymer-based microspheres to achieve three aims at once: embolization, controlled local drug release, and reduced systemic toxicity.12 Two major types of drug-eluting microspheres are available clinically in the United States: LC Beads (Biocompatibles UK Ltd., Farnham, Surrey, United Kingdom) that can be loaded with doxorubicin (DEBDOX) or irinotecan (DEBIRI)13,14 and QuadraSpheres/HepaSpheres (Merit Medical Systems, Inc., South Jordan, Utah) that can be loaded with various drugs including doxorubicin, epirubicin, irinotecan, or cisplatin.15,16 Initially designed for the use in HCC, these products are increasingly used for the treatment of IHC.
Clinical Evidence
One of the very first studies to investigate the safety and feasibility of DEB-TACE in IHC patients used oxaliplatin-eluting microspheres to treat a small collective of nine patients. Patients were prospectively enrolled to receive DEB-TACE associated with continued systemic chemotherapy with oxaliplatin and gemcitabine. A total of 30 DEB-TACE procedures were performed. To assess the true procedure-related toxicity, a retrospective comparison with a group of patients treated only with systemic chemotherapy (N = 11) was performed. As a result, no patient in the DEB-TACE group experience grade 4 toxicities and only very few adverse effects were reported. According to the Response Evaluation Criteria In Solid Tumors (RECIST), 44% of patients achieved partial response and 56% showed stable disease. Most importantly, patients who received both intra-arterial and systemic chemotherapies showed significant survival benefits when compared to chemotherapy alone (OS of 30 and 12.7 months, respectively).17 However, this result must be interpreted with great caution, particularly because of the small sample size. Furthermore, this study was not designed as a prospective randomized trial and patients in the chemotherapy-only group were likely not eligible for intra-arterial therapies because of the very advanced stage of their disease. One of the first true prospective trials, designed to evaluate the safety and efficacy of DEB-TACE in patients with IHC, recruited a total of 11 patients who received a total of 29 individual TACE procedures. Here, each patient received 100 to 150 mg doxorubicin, which appeared to be safe, and no major toxicities were reported. Imaging follow-up was available in all patients, and according to RECIST criteria, all patients were responders and one case demonstrated complete response. The very important aspect of quality of life was addressed, and in 90% of the cases, an improvement was achieved when assessed using the Edmonton Symptom Assessment System questionnaire. As in previous trials and because of the low overall incidence of IHC, the small number of patients in this trial was a significant limitation with regard to survival analysis. Another prospective trial of DEB-TACE in this disease enrolled 26 consecutive patients and was designed to assess feasibility, safety, and efficacy of this approach. Here, irinotecan was used for the loading of DEBs. In addition to the slightly larger number of enrolled patients, the outcome of this trial was retrospectively compared to two independent trials, where a similar cohort of patients received cTACE or systemic chemotherapy. As a result, progression-free survival (PFS) and OS in the DEB-TACE group were 3.9 months and 11.7 months, respectively. When compared with the cTACE cohort (PFS of 1.8 months and OS 5.7 months), DEB-TACE safe and well tolerated and provided a slightly better local tumor control than the other therapies.18 In summary, the data on the use of DEB-TACE for IHC are growing, especially in view of a stronger tumor response when compared to cTACE. However, no prospective randomized trial is available to show clear evidence of survival benefits for DEB-TACE over cTACE. Furthermore, because patients treated with TACE and systemic therapy are living longer, there is now a strong interest in designing prospective randomized trials that would combine TACE with systemic therapy, specifically gemcitabine and cisplatin.
RADIOEMBOLIZATION
Background
Radioembolization exploits the same scientific rationale as TACE and uses the arterial pathway to deliver the payload directly to the tumor. Instead of chemotherapy, however, this modality uses small embolic particles loaded with yttrium 90, a pure β-particle–emitting radionuclide with a half-life of 64.1 hours.19 Currently, there are two types of particles available for clinical use in the United States and Europe: the resin-based SIR-Spheres (SIRTeX Medical Limited, New South Wales, Australia) and the glass-based TheraSpheres (Nordion, Kanata, Ontario, Canada).20 Because of the extremely small size of these particles (ranging from 20 to 60 µm), radioembolization bears the risk of extrahepatic distribution of the aggressive payload via hepatopulmonary shunts. Thus, all patients must be subjected to angiographic evaluation as well as shunt studies using a test injection of 99mTc-labeled macroaggregated albumin (tc-MAA) before the actual procedure.21 The use of radioembolization in liver cancer patients was initially approved in Canada, and only in 1999, the U.S. Food and Drug Administration (FDA) approved this procedure in the United States under the humanitarian device exemption for patients with hepatocellular carcinoma. For most other liver cancer patients including IHC, case-by-case institutional review board approval must be obtained.22
Clinical Evidence
The clinical evidence on radioembolization for IHC is not as comprehensive as the one using cTACE or DEB-TACE. However, a few studies have demonstrated promising results with radioembolization regarding safety and disease control. For instance, a prospective single-center pilot study investigated the feasibility as well as efficacy of radioembolization in 24 patients. All patients had histologically proven diagnosis of IHC. Patients with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2 were eligible for treatment. At the time of the first radioembolization procedure, 38% had imaging signs of portal vein thrombosis (PVT) and a total of 13 patients had multifocal disease. No major life-threatening events were recorded. However, 17% of patients developed grade 3 albumin toxicities and 1 patient developed a refractory gastroduodenal ulcer, itself a characteristic complication of radioembolization. According to EASL response criteria, radioembolization yielded excellent tumor control. Nine percent of the patients achieved complete response and 77% showed partial response on magnetic resonance imaging (MRI). The initial median OS of the entire group was 14.9 months. When stratified according to ECOG performance status, patients with ECOG 0 showed the strongest survival benefits (31.8 months vs. 1 month for ECOG 2).22 This result underlined the impact of the initial patient performance not only for radioembolization but also for all intra-arterial therapies in general. A recently published follow-up study from the same center provided more data and expanded the number of patients to a total of 46. This analysis revealed the potential of radioembolization, showing successful downstaging of the disease to resectable status in 5 patients. The overall median OS in patients without PVT was 14.4 months (5.3 months with PVT).23 Currently, the presence of PVT in candidates for intra-arterial therapies is being discussed, and some evidence indicates slight benefits of radioembolization over TACE in this subgroup of patients.24 However, no final data is available as of today. The results of both studies22,23 were confirmed by another prospective single-center study designed to show the beneficial effects of radioembolization in patients with unresectable IHC. Here, similar survival data (14.7 months for ECOG 1 patients) and comparable toxicity profiles in a cohort of 19 patients were demonstrated.25 Therefore, it is safe to say that radioembolization may also play a beneficial role in the treatment of patients with IHC; however, more prospective randomized trials are needed.
TIPS AND TRICKS
• Use cross-sectional imaging with delayed phases to evaluate the lesions before treatment due to the fibrotic character of the lesions.
• Cholangiocarcinomas are notoriously difficult to visualize angiographically. As a result, it is extremely important to perform cone-beam computed tomography (CT) imaging from various locations to confirm appropriate targeting. This may mean performing several cone-beam CTs during the procedure, especially if the tumor is centrally located and has more than one feeding artery.
• Because most IHC lesions are centrally located, both hepatic arteries must be evaluated even if 90% of the flow is provided by one artery. Angiograms from the right and left hepatic arteries must therefore be performed.
• For radioembolization, inject from a proximal localization and treat in a lobar fashion.
• Use cone-beam CT after the procedure to assess Lipiodol deposition within the tumor after conventional TACE.
• It is better to handle the wire with your fingers rather than a dedicated torque device as they provide true tactile feedback.
• In patients with coagulopathy, closure devices should be considered to reduce the risk of bleeding and hematoma at the puncture site.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree