9 Transrectal Prostate Biopsy
Ahmet Tuncay Turgut and Vikram S. Dogra
Transrectal ultrasound- (TRUS-) guided prostate biopsy is a common outpatient procedure with an annual performance of ∼500,000 in the United States, and is the gold standard for the diagnosis of prostate cancer. Combined use of prostate biopsy and digital rectal examination yields the best diagnostic outcome for prostate cancer. Controversies still exist regarding the optimal number biopsy samples to be taken and their localization. In general, 12 core biopsy of the prostate has replaced the old sextant biopsy.
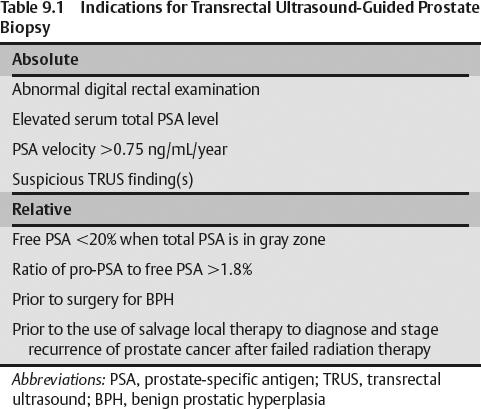
- Abnormal digital rectal examination (DRE), elevated serum total prostate-specific antigen (PSA) levels >4 ng/mL
- A serum PSA velocity higher than 0.75 mg/mL per year and suspicious finding(s) on TRUS examination (Table 9.1)
- Free PSA level less than 20% with serum total PSA level being in the gray zone
- A ratio of pro-PSA to free PSA higher than 1.8%
- Performing the biopsy before surgical treatment of benign prostatic hyperplasia (BPH) and before the use of salvage local therapy to diagnose and stage the recurrence of prostate cancer in patients suspected of failing radiation therapy are further relative indications for prostate biopsy.
- Identify localized cancer in a prostate with an irregular and hard consistency and asymmetric shape (inherently subjective)
- Has high false-negative and false-positive rates
Classically, a total PSA level exceeding 4 ng/mL is accepted as an indication for prostate biopsy, though controversy still exists regarding the upper limit for a normal PSA.
- PSA has similar problems as DRE such as low specificity. Because of its low specificity, the following additional parameters are used to identify high-risk individuals who are to be referred to biopsy:
- Total PSA value of >2–6 ng/mL for patients younger than 50 years of age
- PSA velocity exceeding 0.75 ng/mL
- Free PSA/total PSA ratio less than 0.20 ultrasound features
- Suspicious gray-scale TRUS findings such as hypoechoic nodule, heterogenous echotexture of the peripheral zone (PZ), and hypervascularity detected by color Doppler TRUS are also indications for TRUS-guided prostate biopsy.
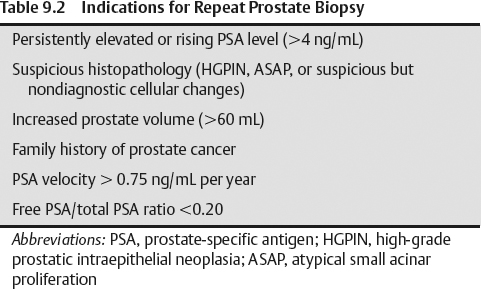
- Increased prostate volume higher than 60 mL
- Family history of prostate cancer
- PSA velocity >0.75 ng/mL per year
- Free PSA/total PSA ratio <0.20
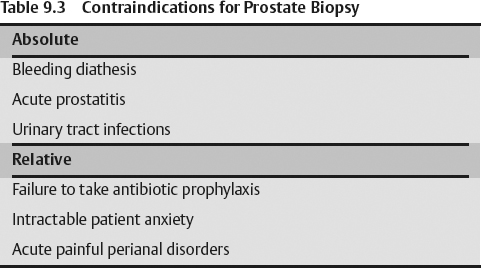
- Bleeding diathesis
- Acute prostatitis
- Urinary tract infections
- Failure to take antibiotic prophylaxis
- Intractable patient anxiety
- Acute painful perianal disorders are considered as relative contraindications.
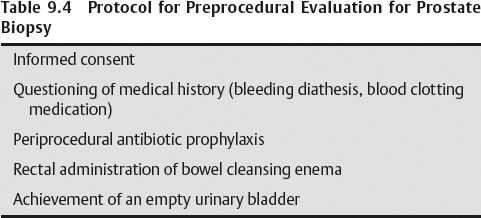
- A written informed consent must be obtained from the patient.
- Medical history of the patient must be questioned for bleeding diathesis and intake of any medication altering blood clotting.
- Discontinuation of anticoagulants, nonsteroidal antiinflammatory drugs 7–10 days prior to procedure
- Stopping of low-dose aspirin (<300 mg per day) is not needed.
- Periprocedural antibiotic prophylaxis of Cipro (ciprofloxacin), 500 mg twice daily beginning before the day of the procedure and continuing for 3 consecutive days
- Request the patient to self-administer a bowel-cleansing enema (precaution against infectious complications)
- Instruct the patient not to empty his urinary bladder completely. It helps define a clear interface with the prostate’s superior margin.
- A color Doppler ultrasound scanner equipped with a transrectal probe is essential for
- TRUS-guided prostate biopsy
- Biplane probes for transrectal prostate scanning with a combination of end-viewing or side-viewing transducers in the 5–8 MHz range
- Use ultrasound gel inside and over a latex condom covering the probe to eliminate the air.
- Anticipate a high level of anxiety in a patient scheduled for TRUS-guided prostate biopsy (worry over pain, discomfort)
- Ensure procedure is performed in an uncrowded, warm, and quiet room