Chapter 24 Tumors of the Uterine Corpus
Malignant tumors of the uterine corpus can be divided into epithelial and mesenchymal types (sarcomas). In 2011, there were an estimated 46,470 cases of cancer involving the uterine corpus in the United States, of which 3% were uterine sarcomas with an estimated 8120 deaths.1,2
I Endometrial Cancer
Introduction
The most common epithelial tumor of the uterine corpus is endometrial cancer, accounting for approximately 50% of all new gynecologic cancers. In the United States, it is the fourth most common malignancy in women, following breast, lung, and colorectal cancers.1
In the majority of women, endometrial cancer is confined to the uterus, as stage I (71%) and stage II (24%) at time of presentation owing to the appearance of abnormal uterine bleeding.3 This clinical presentation and diagnosis allow for a favorable prognosis.
Epidemiology and Risk Factors
In the development of endometrial cancer, age is the most important risk factor. This is a disease predominantly seen in the postmenopausal female, with a median age at diagnosis of 60 years.3 Other risk factors that have been associated in developing endometrial cancer are long-term exposure to unopposed estrogens, residence in North America and Europe, metabolic syndrome (obesity, diabetes), years of menstruation, nulliparity, history of breast cancer, long-term use of tamoxifen, hereditary nonpolyposis colorectal cancer (HNPCC) family syndrome, hormone-replacement therapy with less than 12 to 14 days of progestagens, and first-degree relative with endometrial cancer.4
African American women tend to be diagnosed with higher stage, grade, and risk histologies than non-Hispanic white women.5 Although some cases of endometrial cancer have a hereditary basis, the vast majority are sporadic. HNPCC, Lynch’s syndrome, is an autosomal dominant cancer presenting with early-onset colon, rectal, ovary, small bowel, ureter/renal pelvis, and endometrial cancers. Two to 5% of endometrial cancers are associated with the Lynch syndrome.6 The gynecologic cancer (endometrial or ovarian) presents earlier than colon cancer in about half of the patients with Lynch’s syndrome.4,6,,7
Anatomy and Pathology
On immunohistochemical staining, PTEN mutations are common in type 1 tumors and uncommon in serous tumors. TP53 mutations are not common in type 1 tumors but are common in serous carcinoma.4 Other histologic types of endometrial carcinomas include mucinous, mixed, squamous cell, transitional cell, and undifferentiated.4 Women taking tamoxifen for the treatment of breast cancer are at an increased risk of developing endometrial cancer; however, the risk-benefit ratio favors the use of tamoxifen. Newer aromatase inhibitors are now available and are being used instead of tamoxifen.
Key Points Anatomy and pathology
• Two types of endometrial cancer have been described.
• Type 1 endometrial cancers are the most common, occur in younger women, develop in a background of endometrial hyperplasia and have a favorable prognosis.
• Type 2 endometrial cancers occur in older women, are associated with endometrial atrophy, and are at high risk of relapse and metastases.
Patterns of Tumor Spread
Type 1 tumors primarily spread to pelvic lymph nodes. More positive lymph nodes are seen around the obturator nerve than the external or common iliac vessels. Infrarenal para-aortic adenopathy is seen when there are positive pelvic lymph nodes, adnexal metastases, and serosal infiltration. Type 2 tumors have a higher incidence of lymphatic involvement and extrauterine spread.4 The adnexa and cervix can also be invaded by endometrial carcinoma.
Staging Evaluation
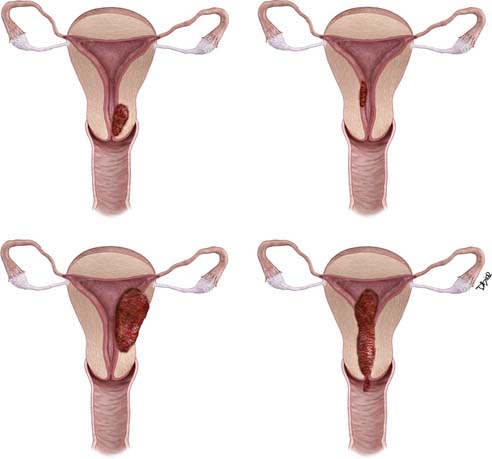
Surgery is used to stage endometrial cancer. The most recent FIGO staging is also used to manage these patients. Stage I is endometrial carcinoma confined to the corpus uteri, whereas stage II involves the corpus uteri and the cervix but has not extended outside of the uterus. Stage III endometrial carcinoma extends outside of the uterus but is confined to the true pelvis. In stage IV, endometrial cancer involves the bladder or bowel mucosa or has metastasized to distant sites. These stages are divided into further subgroups (Table 24-1 and Figure 24-1).8
Table 24-1 AJCC Tumor-Node-Metastases (TNM) and International Federation of Gynecology and Obstetrics (FIGO) Surgical Staging Systems for Endometrial Cancer
| TNM CATEGORIES | FIGO* STAGES | SURGICAL-PATHOLOGIC FINDINGS |
|---|---|---|
| Primary Tumor (T) | ||
| TX | Primary tumor cannot be assessed | |
| T0 | No evidence of primary tumor | |
| Tis† | Carcinoma in situ (preinvasive carcinoma) | |
| T1 | I | Tumor confined to the corpus uteri |
| T1a | IA | Tumor limited to endometrium or invades less than one-half of the myometrium |
| T1b | IB | Tumor invades one-half or more of the myometrium |
| T2 | II | Tumor invades stromal connective tissue of the cervix but does not extend beyond uterus‡ |
| T3a | IIIA | Tumor involves serosa and/or adnexa (direct extension or metastasis)# |
| T3b | IIIB | Vaginal involvement (direct extension or metastasis) or parametrial involvement# |
| IIIC | Metastases to pelvic and/or para-aortic lymph nodes# | |
| IV | Tumor invades bladder and/or bowel mucosa, and/or distant metastases | |
| T4 | IVA | Tumor invades bladder mucosa and/or bowel (bullous edema is not sufficient to classify a tumor as T4) |
| Regional Lymph Nodes (N) | ||
| NX | Regional lymph nodes cannot be assessed | |
| N0 | No regional lymph node metastasis | |
| N1 | IIIC1 | Regional lymph node metastasis to pelvic lymph nodes (positive pelvic nodes) |
| N2 | IIIC2 | Regional lymph node metastasis to para-aortic lymph nodes, with or without positive pelvic lymph nodes |
| Distant Metastasis (M) | ||
| M0 | No distant metastasis | |
| M1 | IVB | Distant metastasis (includes metastasis to inguinal lymph nodes, intra-peritoneal disease, or lung, liver, or bone. It excludes metastasis to para-aortic lymph nodes, vagina, pelvic serosa, or adnexa) |
† Note: FIGO no longer includes Stage 0 (Tis).
‡ Endocervical glandular involvement only should be considered as Stage I and no longer as Stage II.
# Positive cytology has to be reported separately without changing the stage.
Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original and primary source for this information is the AJCC Cancer Staging Manual, Seventh Edition (2010) published by Springer Science and Business Media LLC (SBM). (For complete information and data supporting the staging tables, visit ww.springer.com.) Reprinted from Pecorelli S, Denny L, Ngan H, et al. Revised FIGO staging for carcinoma of the vulva, cervix and endometrium. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2009;105:103-104. Copyright 2009, with permission from International Federation of Gynecology and Obstetrics.
Imaging Evaluation
Primary Tumor
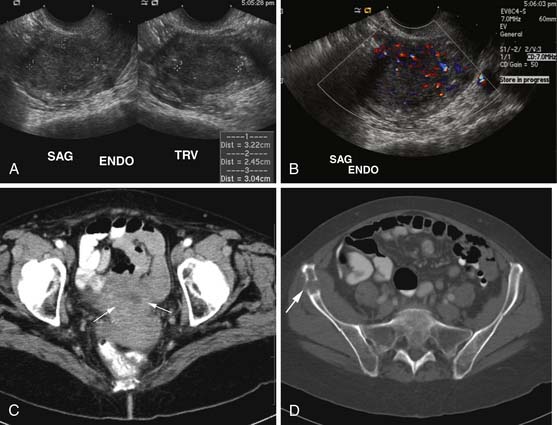
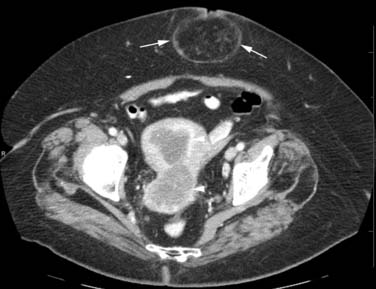
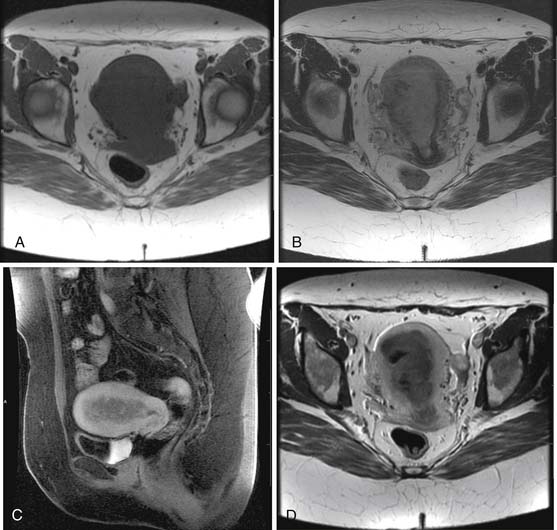
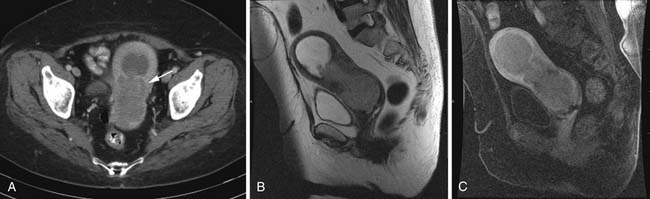
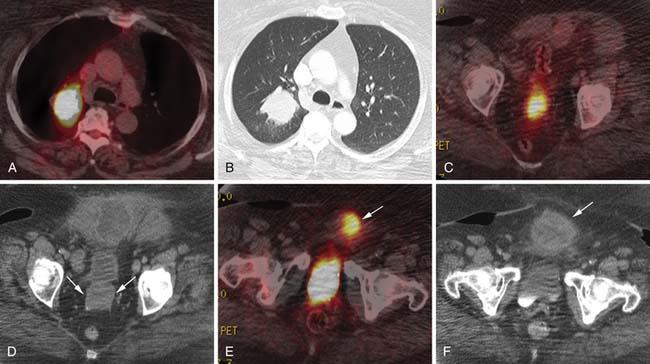
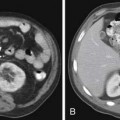
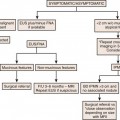
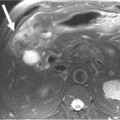
Various imaging modalities (Figures 24-2 to 24-6) can be used to help the attending physician evaluate the patient with endometrial cancer preoperatively, postoperatively, and during surveillance visits.
Ultrasound, especially transvaginal sonography, is noninvasive, is readily available, is less expensive, uses no ionizing radiation, and in some studies, can image the tumor and myometrial invasion, but it can do poorly in evaluating the spread of tumor to adjacent organs.9,10 Endometrial thickness (double layer) greater than 4 to 5 mm in a postmenopausal woman is considered abnormal and raises a flag when there is postmenopausal bleeding. However, the endometrium in this setting is nonspecific and can be seen in hyperplasia, polyps, and cancer.
Magnetic resonance imaging (MRI) is the imaging modality of choice to preoperatively evaluate the depth of myometrial invasion, the presence or absence of adenopathy, and adjacent soft tissue invasion. It has a high soft tissue resolution and accuracy (~90%), especially if it is contrast-enhanced.11,12 However, it is more expensive and, in some locations, is limited in number and availability. This is an ideal imaging modality to help stage a patient who is not a surgical patient.
MRI protocols vary as the number and type of pulse sequences, body orientations, field of view (FOV), matrix and slice thickness, use or lack of use of dynamic imaging, and other factors. Myometrial invasion is best seen with disruption of the junctional zone on T2-weighted images and subendometrial enhancement on dynamic imaging. Adenopathy, cervical and adnexal involvement, and signal change in the bladder wall and rectum can also be seen. In addition, vaginal involvement can be identified. Functional imaging using diffusion-weighted MRI, although not ready for prime time, shows promise and great potential in managing patients with endometrial cancer.13,14
Computed tomography (CT) of the chest and abdomen is best used to evaluate for extrapelvic metastatic disease and possible hydronephrosis. This imaging tool is more readily available, and with newer multidetector systems, the image quality has improved with faster patient throughput; however, it does utilize radiation. More recently, fluoro-2-deoxy-D-glucose (FDG)–positron-emission tomography (PET) and FDG-PET/CT have been used to stage, assist in radiation therapy planning, and evaluate response to therapy and in surveillance. Higher standard uptake values (SUVs) are seen with deep invasion of the myometrium. It is also useful for identifying nodal involvement, given that it may dictate the radiation port.15,16 These imaging modalities are of limited use in diagnosing endometrial carcinoma. Molecular imaging shows great promise for future evaluation in the clinical management of patients with endometrial cancer.
Nodes
Key Points Imaging
• The preoperative imaging evaluation should point out to the surgeon the tumor location, penetration of the myometrium and maybe the cervix, suspected locations of adenopathy, extent into adjacent soft tissues, and any metastatic disease.
• For those patients who undergo chemotherapy, the location of recurrent or metastatic disease and lymphadenopathy needs to be presented to the surgeon and the medical oncologist if the latter is involved with the management of the patient.
• The location of recurrent or metastatic disease and lymphadenopathy can be used for radiation therapy ports.
Treatment
The decision for a lymph node dissection of the pelvis and para-aortic nodal groups, superior and inferior to the inferior mesenteric artery, is made at the time of surgery and is a controversial issue among different centers and geographic regions. In the United States, when the endometrial carcinoma is confined to the uterus, a limited staging procedure is done in that pelvis and para-aortic lymphadenectomy is performed.16
Arguments for and against a pelvic and para-aortic lymphadenectomy are described in the literature with thorough lymph node dissection in patients with type 2 and selected patients with type 1 endometrial cancers.4,17–20
Several trials and various treatment options that use cytotoxic and biologic agents are published in the literature.4,18,21–23
For certain patients, radiation therapy is used to manage their endometrial cancer. The goal of adjuvant radiation therapy is to treat microscopic disease. However, this is controversial worldwide, as is the use of external beam radiation (pelvic radiation, extended field, or whole abdominal radiation) versus vaginal brachytherapy, especially in the high intermediate-risk endometrial carcinomas. Part of the dilemma that leads to controversy is patient selection, experience in grading, and depth of myometrial invasion of these neoplasms by various pathologists.4,24–27
Key Points Therapy
• Surgery is the standard of treatment for those surgical candidates with endometrial cancer.
• Radiation therapy is used in the postoperative state to manage recurrence and adenopathy.
• Chemotherapy is used for palliation; with newer biologic agents, a longer period of survival with less toxicity may be possible.
New Therapies
The mainstay of therapy for endometrial cancer is surgery followed by radiation therapy and chemotherapy. Advances have been made into the understanding of the molecular biology of endometrial cancer.21–23,28 These discoveries have clinical and therapeutic implications. Some of the genetic alterations seen with endometrial cancer involve among others the tumor suppressor protein PTEN and associated pathways, microsatellite instability, K-ras mutations, p53 mutations, and HER-2/neu expression. Some of the target therapies have been directed to the mammalian target of rapamycin (mTOR) inhibitors, human epithelial growth factor receptor (EGFR), and angiogenesis inhibition of the vascular endothelial growth factor (VEGF). With time, these target therapies and others to be discovered should lead to better patient care and survival and minimizing postoperative therapeutic complications. A full discussion on these therapies is beyond the scope of this chapter.