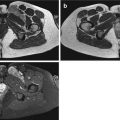
Fig. 18.1
Intramuscular myxoma at the right scapular region in a 68-year-old man. (a) Axial spin-echo T1-weighted MR image. (b) Axial spin-echo T2-weighted MR image. (c) Axial spin-echo T1-weighted MR image after gadolinium contrast injection. A large, rounded, intramuscular lesion at the infraspinatus fossa is visible. The mass is well circumscribed and homogeneous on both spin-echo sequences, hypointense to muscle tissue on the T1-weighted image (a), and hyperintense to fat on the T2-weighted image (b). After contrast medium injection (c), only a few enhancing strands are observed within the lesion. The major parts of the lesion do not enhance. This pattern of enhancement illustrates well the sparse vascularization of the lesion
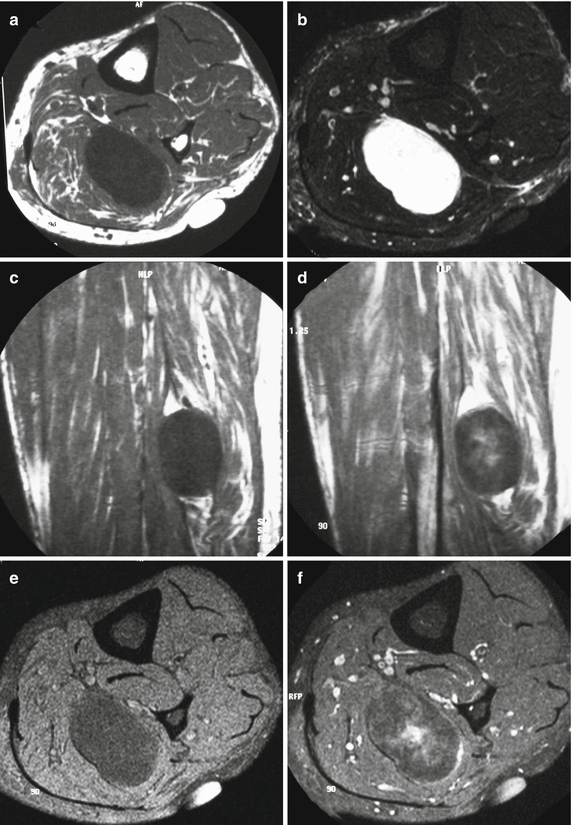
Fig. 18.2
Intramuscular myxoma of the calf. (a) Axial spin-echo T1-weighted MR image. (b) Axial STIR T2-weighted MR image. (c) Sagittal spin-echo T1-weighted MR image. (d) Sagittal spin-echo contrast-enhanced T1-weighted MR image. (e) Axial spin-echo T1-weighted MR image with fat suppression. (f) Axial spin-echo T1-weighted MR image with fat suppression after gadolinium contrast injection. Oval-shaped mass within the soleus muscle is observed. The lesion is sharply outlined. On T1-weighted images, the lesion is hypointense to muscle tissue (a, c, e). T2-weighted images reveal a homogeneously hyperintense aspect of the tumor, with signal intensity equivalent to fluid (b). Following injection of contrast medium, no enhancement is observed at the periphery of the lesion, while the central part shows a definite enhancement (d, f). The areas of enhancement correspond to regions with high amount of solid myxoid tissue
Fig. 18.3
Multiple intramuscular myxomas with polyostotic fibrous dysplasia of the bones of the lumbar spine, pelvic bones, and femur in a 35-year-old man (Mazabraud’s syndrome). Plain radiograph discloses multiple multilocular cyst-like lesions with a ground-glass appearance, separated from each other by thin septations within the fifth lumbar vertebra, the right pelvic bones, and the right femur, corresponding to fibrous dysplasia. These involved bones are markedly deformed. Endomedullary osteosynthetic material at the femur witnesses the previous pathologic fracture (Courtesy of Trigaux JP, Nisolle JF, Cliniques Universitaires UCL de Mont-Godinne, Belgium)
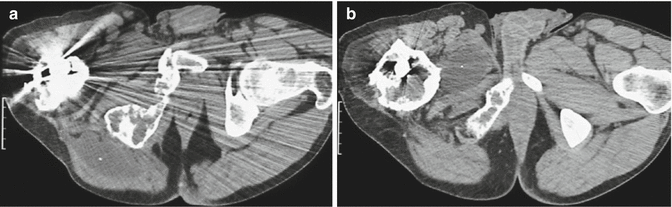
Fig. 18.4
Multiple intramuscular myxomas with polyostotic fibrous dysplasia of bone of the lumbar spine, pelvic bones, and femur in a 35-year-old man. (a) CT at the level of the hips. (b) CT at the level of right trochanteric region. CT confirms the replacement of medullary fat of the right ischium and femur by fibrous tissue. Expansion and thickening of the overlying cortex. These findings correspond to fibrous dysplasia of the bone. In addition, well-delineated, non-enhancing, homogeneously hypodense soft tissue masses are observed within the right gluteal (a) and adductor (b) muscles, corresponding to intramuscular myxomas (Courtesy of Trigaux JP, Nisolle JF, Cliniques Universitaires UCL de Mont-Godinne, Belgium)
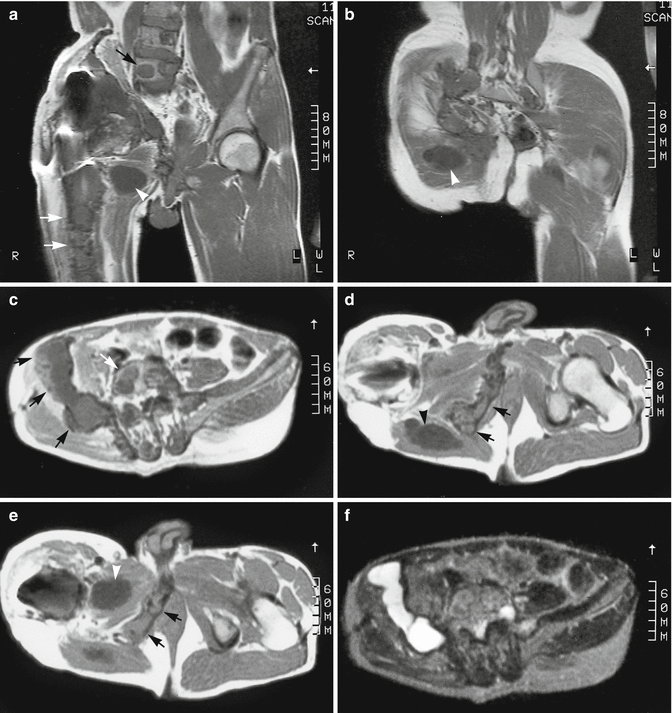
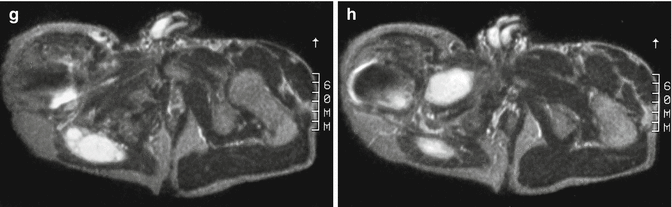
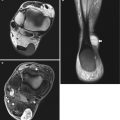
Fig. 18.5
Multiple intramuscular myxomas with polyostotic fibrous dysplasia of bone of the lumbar spine, pelvic bones, and femur in a 35-year-old man. (a, b) Coronal spin-echo T1-weighted MR images at the level of the right femoral diaphysis (a) and gluteal muscles (b). (c–e) Axial spin-echo T1-weighted MR images at the level of the iliac wings (c), hips (d), and trochanteric regions (e). (f–h) Axial spin-echo T2-weighted MR images at the level of the iliac wings (f), hips (g), and trochanteric regions (h). The intramuscular myxomas appear as homogeneous, very low-intensity soft tissue masses within the right adductor (a, e) and gluteal (b, d) muscles (arrowheads). The areas of the right pelvic bones, right femur, and lumbar vertebrae that are involved by fibrous dysplasia have a lobular appearance with low signal intensity (arrows). On T2-weighted images, both myxomas are very bright, indicating long T2 relaxation times of their myxoid matrix. The gluteal myxoma is composed of several lobules, separated from each other by low-intensity septations. The areas involved by fibrous dysplasia of the bone are best observed at the level of the right iliac wing. Due to cystic degeneration, these areas appear extremely bright on T2-weighted images. Artifacts are observed on all images in the right hip region. These are due to magnetic field inhomogeneity, which is caused by the metallic hip prosthesis (Courtesy of Trigaux JP, Nisolle JF, Cliniques Universitaires UCL de Mont-Godinne, Belgium)
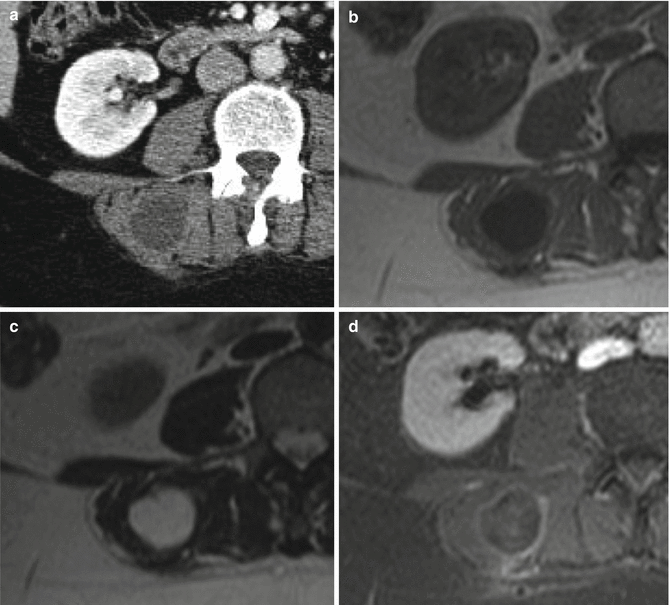
Fig. 18.6
Intramuscular myxoma in the autochthonous back muscles. (a) Axial CT-scan. (b) Axial T1-weighted MR image. (c) Axial T2-weighted MR image. (d) Axial T1-weighted MR image after gadolinium contrast injection. On CT, the myxoma presents as a well-demarcated lesion within the skeletal musculature with an intermediate attenuation (i.e., between that of water and muscle tissue) (a). Typically, one can see hypointensity on T1-weighted images (b), very high intensity on T2-weighted images (c), as well as inhomogeneous contrast enhancement (d)
18.3.1.4 Imaging Strategy
Conventional radiographs are of little value for the diagnosis of intramuscular myxoma. An exception is made in the case of patients with both multiple myxomas and fibrous dysplasia, but in these cases, conventional radiographs are used primarily for investigation of the bony lesions (Fig. 18.3). Of note, Mazabraud’s syndrome should always be taken into account when fibrous dysplasia occurs with one or more soft tissue masses. CT – like MRI – may reveal misleading findings by demonstrating fat densities within a myxoma that suggest a lipomatous tumor (lipoma or well-differentiated liposarcoma). Sarcomas, particularly with myxoid degeneration, may resemble myxomas both radiologically and histologically. Although MRI is the preferred imaging technique for staging, one has to admit that the MRI findings of intramuscular myxoma are not highly specific.
Synovial cysts, bursas, and ganglia, which can look like a myxoma, are usually juxta-articular and intermuscular and not intramuscular. Moreover, as cystic masses, they show a thin rim of enhancement after administration of intravenous contrast agent. Neurogenic tumors (e.g., neurofibroma, nerve sheath tumors) are also rather inter- than intramuscular, and often a so-called target sign is seen on T2-weighted MR images [117].
18.3.2 Acral Fibromyxoma
(Superficial) Acral fibromyxoma (AFM) is a very rare benign tumor arising in the subungual and periungual regions in fingers and toes of adult patients usually not exceeding 2 cm in diameter. Men are more frequently affected by the slow-growing and painless lesion than women (2:1). Histologically, AFM consists of spindled fibroblast-like and stellate cells surrounded by a myxoid and/or collagenous matrix with immunoreactivity for CD34, CD99, endomysial antibody (EMA), vimentin, and CD10. After surgical excision which is the treatment of choice, recurrence is rare [9, 52].
AFM has been described as being isointense to the muscle on T1-weighted MR images, while being hyperintense on T2-weighted images. This imaging appearance distinguishes AFM from giant cell tumors of the tendon sheath, which are predominantly hypointense on T1- and T2-weighted sequences. Homogeneous enhancement is seen on post-contrast MRI. When located in deeper tissues with contact to the bone, osseous erosions or scalloping can be caused [16, 156] (Fig. 18.7).
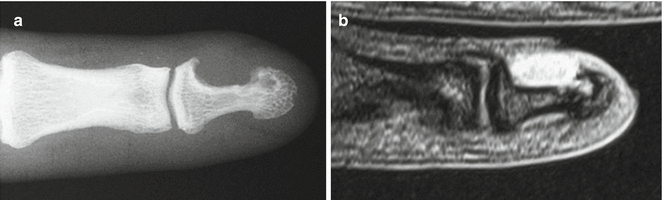

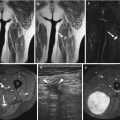
Fig. 18.7
Acral fibromyxoma. 32-year-old Caucasian male with a acral fibromyxoma of the tip of his right index finger (a) Radiography, (b) Coronal T2-weighted MR Image. Antero-posterior X-ray (a) demonstrates scalloping of the outer cortical margin of the distal phalanx, with overhanging edges and radiolucency distally, suggesting intraosseous extension. Coronal T2 MRI (b) reveals a T2 hyperintense lesion along the cortical margin of the phalanx, with a small component distally, extending into the medulla (Used with permission from Varikatt et al. [156])
18.3.3 Juxta-Articular Myxoma
Juxta-articular myxomas are characterized by depositions of myxoid material in the juxta-articular tissues of the knee or rarely other joints and occasionally with myxoid changes within the underlying cartilage. The term “parameniscal cyst” refers to small types of such lesions. Calcification of the myxoid matrix may occur in older lesions [5, 159].
18.3.4 Myxoma of the Jaws
Although primarily a bone tumor, myxoma of the jaws (odontogenic myxoma) may manifest as a myxomatous swelling or mass in the soft tissues near the mandible or maxilla. It is characterized by higher cellularity and cellular pleomorphism than other myxomas.
Most myxomas of the jaws affect young adults. In its common presentation, the tumor overlies an osteolytic defect in the mandible and/or maxillary bone and may displace or destroy the teeth, penetrate into the maxillary sinus, or involve soft tissues of the face.
Myxoma of the jaws is seen radiologically as an expansive, well-circumscribed, most often multilocular radiolucency within the jaw bone. CT is recommended for assessing the extent of the lesion (Fig. 18.8). MRI is superior for demonstrating the extent of soft tissue involvement, but is inferior to CT for evaluating the underlying bony lesion [29, 83, 86, 99] (Figs. 18.8 and 18.9). The tumors are usually hyperintense in T2-weighted MR images and show contrast enhancement in the peripheral areas, while the center does not enhance [86].
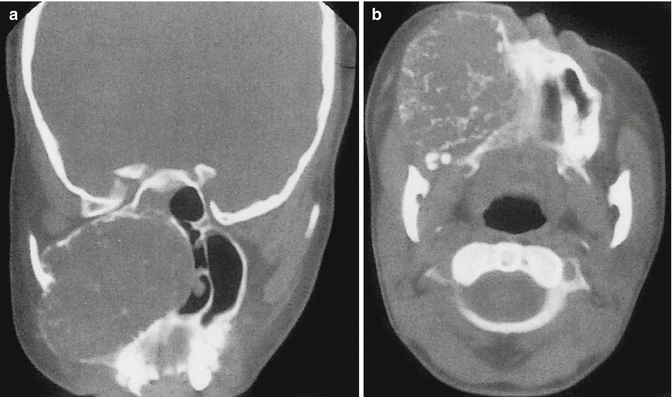
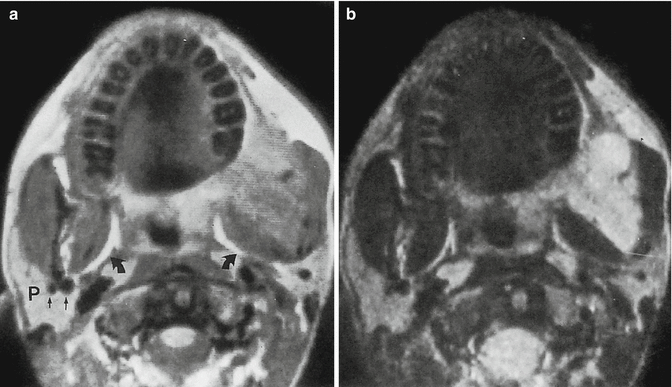

Fig. 18.8
Myxofibroma of the jaws in an 18-year-old woman. (a) Coronal CT. (b) Axial CT. The presence of an expansive myxofibroma replacing the right maxillary antrum. Expansion, thinning, and destruction of the surrounding bony margins are obvious. Bony trabeculations are well seen within the lesion (Reproduced from [29], with permission)

Fig. 18.9
Myxofibroma of the jaws in a 16-year-old boy. (a) Axial spin-echo proton density-weighted MR image. (b) Axial spin-echo T2-weighted MR image. Expansive lesion at the left ascending ramus of the mandible is seen. On the proton density-weighted image, the myxofibroma has increased signal intensity compared with the pterygoid and masseter muscles. The T2-weighted image reveals high signal intensity of the tumor, surpassing that of fat. Tumor margins are sharp. Note the bright signal of the parapharyngeal fat (curved arrows) and the presence of vascular structures (straight arrows) within the parotid gland (P) (Reproduced from [29], with permission)
18.3.5 Deep (“Aggressive”) Angiomyxoma
18.3.5.1 Definition
18.3.5.2 Incidence and Clinical Behavior
The majority of angiomyxomas occur in women between ages of 25 and 60 years. This tumor has a predilection for the gluteal, perineal, and pelvic regions. Although a slow growth pattern is seen, the tumor is focally infiltrative, often extending into the paravaginal and perirectal regions. Metastatic spread is not observed. Recurrence rate after surgical removal is high [100].
18.3.5.3 Imaging
On CT, aggressive angiomyxoma has a variable appearance. It has been described either as a predominantly cystic mass containing solid components or as a solid mass containing low-density areas [100, 163]. MRI reveals a high signal intensity mass with hypointense strands of fibrovascular tissue on T2-weigthed images. In addition, mild restriction on diffusion-weighted imaging (DWI) and high mean values in apparent diffusion coefficient (ADC) maps as well as a layered enhancement seem to be typical [113]. The lesion shows a tendency to displace pelvic organs and to grow around pelvic floor muscles, but without disrupting them (Fig. 18.10). Of note, the lack of high fat content is a key feature in differentiating aggressive angiomyxoma from myxoid liposarcoma.
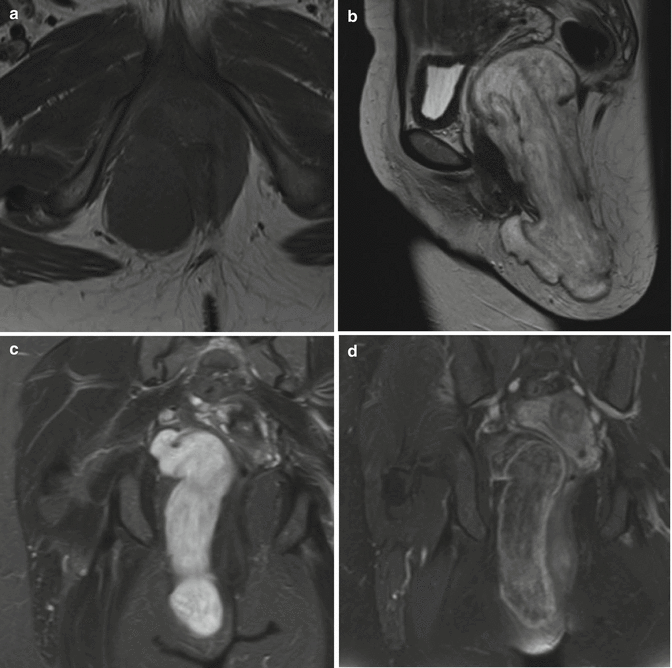

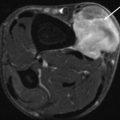
Fig. 18.10
Paravaginal aggressive angiomyxoma. (a) Axial T1-weighted MR image. (b) Sagittal T2-weighted MR image. (c) Coronal T2-weighted MR image with fat saturation (fs). (d) Coronal T1-weighted MR image after gadolinium contrast injection with fs. The paravaginal aggressive angiomyxoma which measured more than 20 cm and displaced the uterus and bladder was initially misinterpreted as bartholinitis. On T1-weighted images, the mesenchymal tumor presents as a hypointense mass to muscle tissue (a), while showing a high signal intensity on T2-weighted images (b, c) with typical hypointense strands of fibrovascular tissue, which also enhanced after gadolinium contrast injection (d)
18.3.6 Pleomorphic Hyalinizing Angiectatic Tumor
18.3.6.1 Definition
Pleomorphic hyalinizing angiectatic tumor (PHAT) is a very rare entity of low malignant potency which occurs in the subcutaneous tissues of the lower extremity. Grossly, the lesions have a lobulated appearance with characteristic thin-walled angiectatic vessels surrounded by fibrin and collagen [59, 142].
18.3.6.2 Incidence and Clinical Behavior
Pleomorphic hyalinizing angiectatic tumors occur mainly in the fifth decade, and men are affected more frequently. Usually, these tumors are found in the superficial subcutaneous fat tissue of the trunk, as well as the upper and predominantly the lower limb. Recurrence rate after surgical removal is high; metastases have not been reported [100, 110].
18.3.6.3 Imaging
On pre-contrast CT scans, the tumor is inhomogeneously hypodense. After contrast administration, PHAT usually shows a heterogeneous enhancement with lower density around the angiectatic vessels corresponding to the hyalinized vessel walls [92]. MRI can illustrate the ill-defined margins and the infiltration of fat tissue; beyond this, MRI findings are rather unspecific, since PHAT can show solid and cystic components and variable contrast enhancement [146] (Figs. 18.11, 18.12 and 18.13).
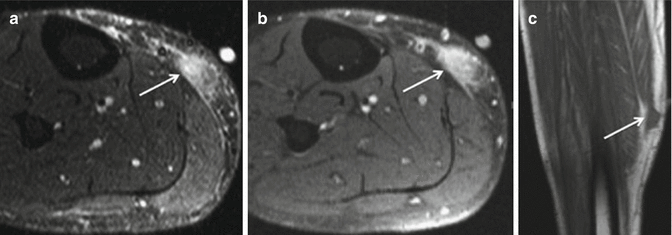
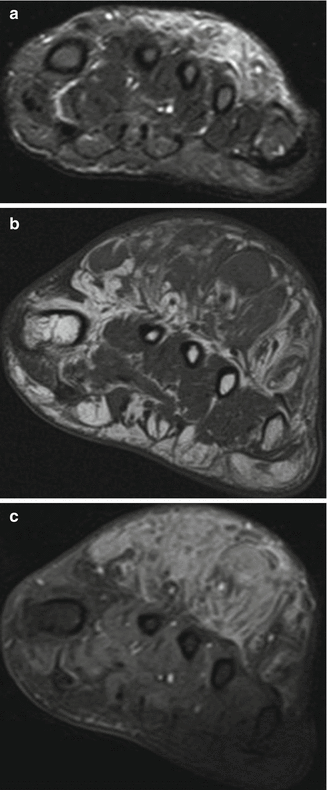
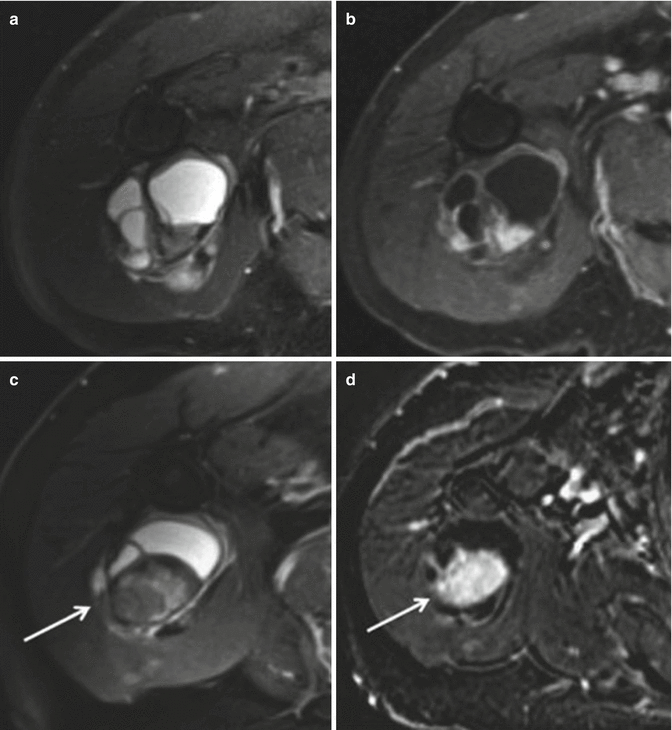

Fig. 18.11
A 46-year-old male with pleomorphic hyalinizing angiectatic tumor in the calf. (a) Axial T2-weighted MR image (STIR). (b) Axial T1-weighted MR image post-contrast. (c) Coronal T1-weighted image. The STIR image (a) shows a subcutaneous hyperintense lesion (arrow) overlying the medial soleus and medial head of the gastrocnemius muscles. Axial post-contrast image (b) at the same level shows relatively homogeneous enhancement of the subcutaneous mass (arrow), which abuts the underlying gastrocnemius muscular fascia but remains confined to the subcutaneous soft tissues. Note ill-defined margins and strands of signal abnormality extending along the fascia. Coronal T1-weighted image (c) demonstrates homogeneous T1 isointensity to muscle (arrow), with minimal infiltration of the subcutaneous fat (Used with permission from Subhawong et al. [146])

Fig. 18.12
49-year-old female with PHAT in the foot. (a) Axial proton density–weighted MR-image with fat saturation. (b) Short axis T1-weighted MR-image. (c) Short axis T1-weighted post-contrast image with fat saturation. Proton density–weighted images (a) with fat saturation shows ill-defined hyperintense signal in the subcutaneous tissues at the dorsum of the forefoot over the metatarsal shafts. Short axis T1-weighted image (b) from subsequent MRI 3 years later demonstrates significant interval growth of the ill-defined soft tissue mass. Short axis post-contrast image (c) demonstrates diffuse enhancement within the mass on the later exam. Note interspersed fat signal throughout the lesion and ill-defined margins

Fig. 18.13
An 87-year-old woman with PHAT of the proximal upper extremity. (a) Axial T2-weighted MR-image with fat saturation. (b) Axial T1-weighted MR-image post-contrast with fat saturation. (c) Axial T2-weighted MR-image with fat saturation. (d) Axial T1-weighted MR-image post-contrast with fat saturation. Axial T2-weighted (a) and post-contrast T1-weighted images (b) performed at presentation demonstrate a multiloculated cystic mass in the right triceps muscle, with nodular and thickened enhancing septa. Axial T2-weighted (c) and post-contrast T1-weighted images (d) from subsequent examinations 2.4 years later show an enlarging solid component (arrows) within the cystic portion of the mass. The mass was later resected, and histologic features were most suggestive of a PHAT (Used with permission from Subhawong et al. [146])
18.3.7 Ectopic Hamartomatous Thymoma
18.3.7.1 Definition, Incidence, and Clinical Behavior
Ectopic hamartomatous thymoma (EHT) is an extremely rare benign tumor usually located in the lower neck region next to the sternocostal joint. Predominantly, men in the third and fourth decade are affected. These tumors are well defined and slow growing, not showing metastasis. Histologically, these tumors consist of spindle cells and epithelial and adipose cell elements [135]. Only seldom recurrence after resection is seen.
The histogenesis remains unclear: initially, it was thought that EHT could derive from ectopic thymus tissue along the migration path of the embryonic thymus from the pharynx to the anterior mediastinum, but since normal thymus tissue never was found in EHT, investigators now believe that it could be of branchial origin, respectively, a remnant of the submerged caudal portion of the second arch and the third and fourth branchial arch [51].
18.3.7.2 Imaging
Until the present, no specific study concerning imaging has been performed. Thus, when encountering a space-occupying lesion in the lower neck region next to the sternocostal joint in men, EHT should be incorporated into the differential diagnostic list.
18.4 Intermediate Tumors (Locally Aggressive)
18.4.1 Hemosiderotic Fibrolipomatous Tumor
18.4.1.1 Definition, Incidence, and Clinical Behavior
Hemosiderotic fibrolipomatous tumor (HFLT) is a rare soft tissue neoplasm consisting of adipocytes, hemosiderin pigments, and fibrous tissue. The tumor arises characteristically in the foot or ankle region or the dorsum of hands of middle-aged women and tends to recur in 30–50 % after excision [54]. Malignant transformation into myxoinflammatory fibroblastic sarcoma has been described [143], an entity with the same characteristic chromosome translocation t(1;10) (p22;q24) as HFLT.
18.4.1.2 Imaging
Radiography and ultrasound are usually nonspecific. MRI shows an often ill-defined tumor with fatty areas divided by reticular septations. On T1-weighted sequences, HFLT is iso- or hypointense to the muscle, while being hyperintense on T2-weigthed images, possibly due to the myxoid/fibrous content of HFLT. Contrast enhancement of the nonfatty tumor parts has been described (Fig. 18.14). Because of hemosiderin deposition in HFLT, susceptibility-weighted imaging sequences such as T2* can show characteristic blooming in foci of hemorrhage, which can be helpful to distinguish HLFT from other lipomatous lesions [121].
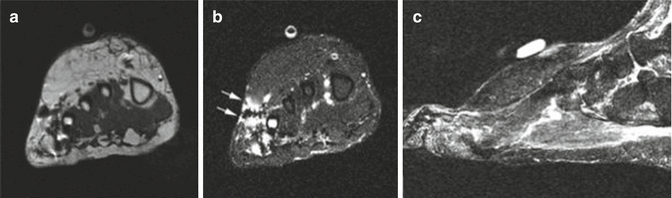

Fig. 18.14
56-year-old woman with haemosiderotic fibrolipomatous tumorsuperficial to the metatarsals. (a) Coronal T1-weighted MR image. (b) Coronal T2-weighted MR image with fat saturation. (c) Sagittal T2-weighted fat-suppressed MR image. The coronal T1-weighted MR image (a) shows a high-signal mass superficial to the metatarsals that is predominantly fat-intense. A coronal T2-weighted fat-suppressed MR image (b) shows a predominantly fat-intense mass. Also present, as indicated by the arrows, are unrelated areas of high and low signal intensity that represent postsurgical changes from the patient’s prior foot surgeries. A sagittal T2-weighted fat-suppressed MR image again shows a fat-intense mass superficial to the metatarsals (c). (Used with permission from Moretti et al. [165])
18.5 Intermediate Tumors (Rarely Metastasizing)
18.5.1 Atypical Fibroxanthoma
18.5.1.1 Definition, Incidence, and Clinical Behavior
Atypical fibroxanthoma (AFX) is a rare mesenchymal tumor of intermediate malignancy affecting elderly patients and arising in the sun-exposed cutis of the head and neck region. AFX tends to show exophytic growth; ulceration and bleeding are also possible. Histologically, AFX consists of fibroblastic spindle cells as well as multinucleated epithelioid cells and giant cells. Despite the sometimes aggressive histological appearances, AFX has a benign clinical course, when diagnosed early and excised completely [89, 105].
18.5.1.2 Imaging
There is only limited literature about the radiological appearance of AFX available. Ultrasonography and MRI can be used to determine the extent of the tumor or possible infiltration of subcutis. In one case study, AFX shows intermediate signal intensity on T1-weighted and T2-weighted images and diffusely heterogeneous enhancement after contrast-agent administration [96], while both malignant melanoma and squamous cell carcinoma as possible differential diagnoses tend to have a high signal intensity on T2-weighted images. Melanin within melanoma may also cause elevated T1 signal intensities.
18.5.2 Angiomatoid Fibrous Histiocytoma
18.5.2.1 Definition
Angiomatoid fibrous histiocytoma (AFH) is a rare, low-malignant soft tissue tumor. Usually, AFH presents as a well-circumscribed multilobular mass showing a fibrous pseudocapsule with typical lymphocytic infiltrates and consisting of fibroblast and histiocyte-like cells of low mitotic activity and blood-filled spaces [82].
18.5.2.2 Incidence and Clinical Behavior
AFH usually occurs in children and young adults. It predominantly affects the dermis and subcutaneous tissue in the extremities and the trunk. Metastasis is only rarely seen.
18.5.2.3 Imaging
Correspondingly, CT and MRI show a lobulated soft tissue mass sometimes with fluid–fluid levels and focal low signal intensity in T1- and T2-weighted sequences as a sign of intralesional hemorrhage [97] (Fig. 18.15).
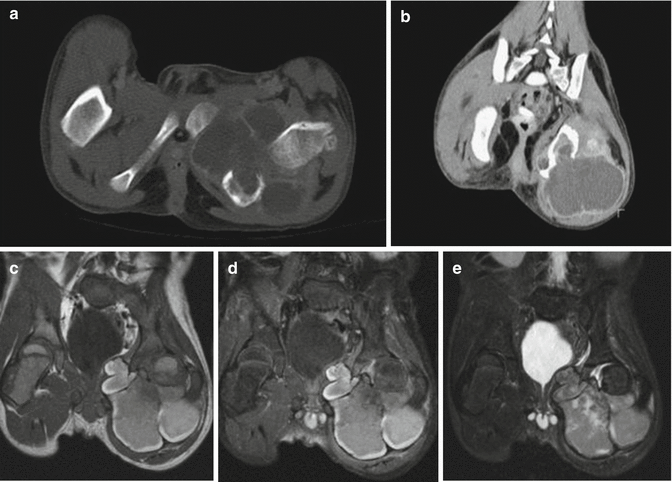

Fig. 18.15
A 5-year-old boy with AFH of the bone. (a, b) Axial and coronal CT. (c) Coronal T1-weighted MR image. (d) Coronal T1-weighted gadolinium-enhanced fat-saturated MR image. (e) Coronal STIR MR-image. Axial and coronal reformat CT scan images demonstrate an expansile lytic lesion with a large cortical break in the left posterior ischium (a). The mass is predominantly fluid-density with thin, enhancing septations, and an extensive extraosseous component. Coronal T1-weighted MR image demonstrates a lobulated intraosseous left ischial mass with hyperintense peripheral signal (felt to represent methemoglobin) as well as an extensive extraosseous component (c). A fine dark signal capsule or pseudo-capsule is also noted beyond the hyperintense rind. Coronal T1-weighted gadolinium-enhanced fat-saturated image demonstrates at most minor enhancement with much of the peripheral hyperintensity present pre- contrast. The fine dark signal rind is again noted (d). Coronal STIR image demonstrates heterogenous signal intensity and a dark rim (e). (Used with permission from Petrey et al. [166])
18.5.3 Ossifying Fibromyxoid Tumor
18.5.3.1 Definition
Ossifying fibromyxoid tumor (OFMT) is a rare soft tissue tumor of still unknown origin arising in the subcutaneous tissue of the extremities usually following a benign clinical course. Yet single cases showing cellular atypia, aggressive behavior, and metastasis have been described [87]. Typically, OFMT consists of small round cells and osteoid or lamellar bone [44, 58, 87, 136].
18.5.3.2 Incidence and Clinical Behavior
OFMT presents as a slowly growing lobulated, ossified, and encapsulated mass in the fifth to seventh decade of life with a male predominance. The tumor diameter does seldom exceed 10 cm.
18.5.3.3 Imaging
CT can demonstrate the characteristic lamellar bone formation as well as a strong contrast enhancement corresponding to the good vascularization that is typical of OFMT. In MRI, focal areas of high signal intensity on T1-weighted images of the tumor as a sign of fatty marrow spaces seem to be characteristic [67, 125] (Fig. 18.16).
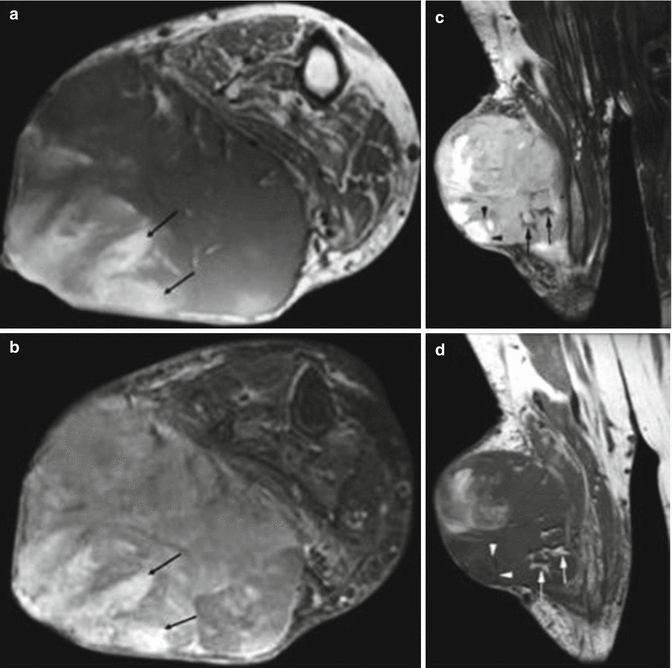

Fig. 18.16
77-year-old woman with ossifying fibromyxoid tumor of the calf. (a) Axial T1-weighted MR image. (b)Axial T2-weighted MR image with fat saturation (FS). (c) Coronal T2-weighted MR image. (d) Coronal T1-weighted MR image. Axial T1W (a) and T2W FS (b) images through the right calf demonstrate the mass, which is subcutaneous and seen to displace, rather than infiltrate, the adjacent musculature. The mass is predominantly isointense to muscle on T1W image and of intermediate-to-high signal intensity on T2W FS image. Foci of hyperintensity are seen on the posterolateral aspect of the mass on both sequences (arrows), in keeping with haemorrhage. Coronal MR images show areas of low signal intensity (black arrows) on T2W FS (c) and high signal intensity (white arrows) on T1W (d) sequences, consistent with fatty marrow spaces. There are also foci consistent with necrosis, seen with areas of low signal intensity on T1W (white arrow heads) and high signal intensity on FSE T2W FS (black arrow heads) images. (Used with permission from Harish et al. [67])
18.5.4 Mixed Tumor
18.5.4.1 Myoepithelioma
Definition
Myoepithelioma is a benign tumor showing a wide range of histopathological features making the diagnosis complicated [35]. Tumors have a multinodular or lobular appearance and can be well circumscribed or infiltrative. Spindled, ovoid, or epithelioid tumor cells are seen, while atypia is absent.
Incidence and Clinical Behavior
Imaging
A search of the literature reveals no specific reports on imaging findings. Thus, subcutaneous localization is the only diagnostic clue (Fig. 18.17).
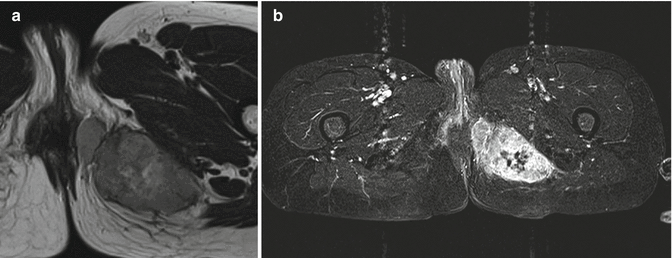
Fig. 18.17
Myoepithelioma of the left perineal/gluteal region. (a) Axial T1-weighted MR image. (b) Axial T1-weighted MR image with fat saturation and subtraction after gadolinium contrast injection. A 50-year-old patient with a malignant myoepithelioma of the left perineal/gluteal region. On T1-weighted images, the tumor is slightly hyperintense to the muscle (a), and after contrast-agent administration, a strong peripheral enhancement with a central non-enhancing necrotic area can be noted (b)
18.5.4.2 Parachordoma
Definition
The term “parachordoma” has been applied to a rare tumor of uncertain histogenesis but with a characteristic histologic appearance with vacuolated eosinophilic epithelioid cells. The tumor forms a lobulated mass with diameter averaging 3.5 cm. Usually, it involves the soft tissues of the extremities adjacent to tendons, synovium, or bones [3, 33, 53].
Incidence and Clinical Behavior
Parachordoma is a rare tumor, which affects both adolescents and adults. The lesion predominates in the extremities or peripheral parts of the body where it is deeply seated near tendons, synovium, and osseous structures [53]. Although recurrence has been observed following excision, parachordoma is considered a benign lesion [55].
Imaging
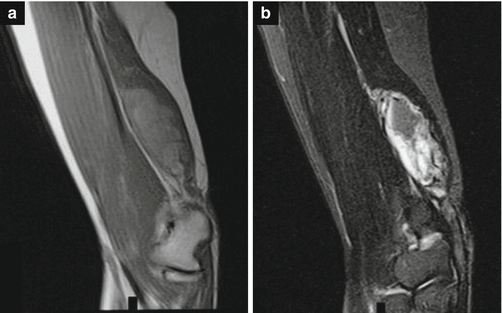
Fig. 18.18
60-year-old woman with parachordoma of the elbow. (a) Sagittal T1-weighted MR image. (b) Sagittal T2-weighted MR image. Sagittal MRI of the distal upper arm soft tissue mass shows a heterogeneous dark signal on the T1-weighted image (a) and a heterogeneous bright signal on the T2-weighted image (b). (Used with permission from Clabeaux et al. [28])
18.5.5 Phosphaturic Mesenchymal Tumor
18.5.5.1 Definition, Incidence, and Clinical Behavior
Phosphaturic mesenchymal tumor (PMT) is a rare neoplasm that causes systemic phosphate depletion and consequently an oncogenic osteomalacia by producing a phosphaturic substance, the so-called fibroblast growth factor 23 (FGF23). The tumor can occur in the soft tissue and bone; in almost half of the cases, the lower extremities are affected. There is no sex predilection and the age range of patients is wide. Clinically, muscle weakness, bone pain, and multiple fractures as well as renal phosphate wasting and elevated serum levels of FGF23 are characteristic. The tumor itself is a slow-growing ill-defined mass and can histologically be divided in three variants: osteoblastoma-like, non-ossifying fibroma-like, and the most common mixed connective tissue variant (PMTMCT). After complete excision, bone consistency and serum levels of fibroblast growth factor 23 get back to normal [48, 84].
18.5.5.2 Imaging
Since PMT usually do not exceed a diameter of 5 cm and can be located ubiquitary, diagnosis can be challenging. Radiography and CT scans often only show a general osteopenia and in case of intraosseous location lytic lesions with or without mineral deposition. Whole-body MRI is more helpful in finding the lesions, with T1 intensity depending on the vascularization and hyperintensity on T2-weighted images [48, 118]. In addition, whole-body scintigraphy with 111In-octreotide and 68Ga-DOTANOC PET/CT and 68Ga-DOTATATE PET/CT is also excellent in detecting PMT [50] (Fig. 18.19).
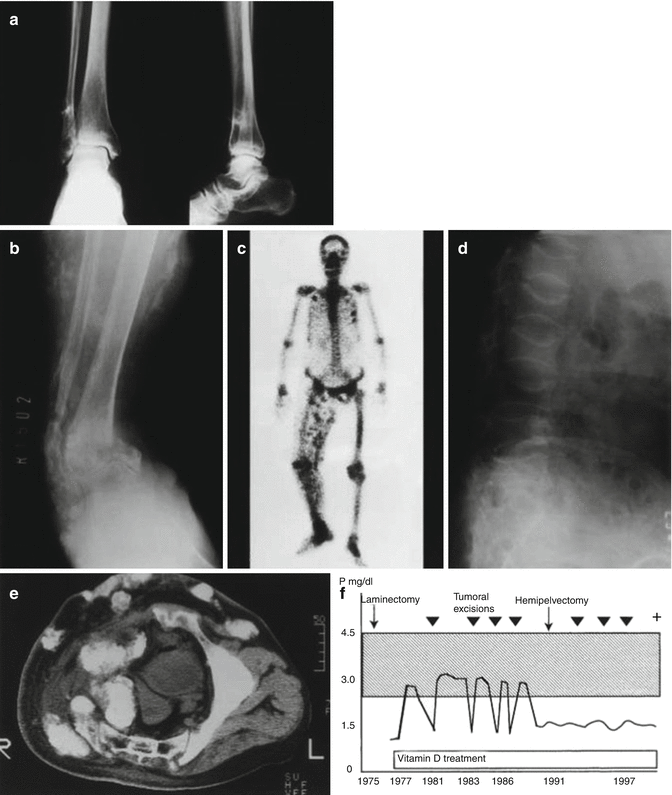

Fig. 18.19
45-year-old woman with phosphaturic mesenchymal tumor. (a) Radiographs showing a mineralized juxtacortical tumor adjacent to the right distal fibula. The tumor was originally diagnosed as exostosis and ignored. (b) Radiograph 14 years later showing multiple mineralized soft tissue tumors in the right lower leg with pathological fractures of the tibia and fibula. (c) Bone scan in 1991 showing multiple soft tissue masses in the right lower extremity and fractures of the right ankle and ribs. (d) Radiograph showing multiple spinal compression fractures due to uncontrollable osteomalacia. (e) Computed tomography scan showing mineralized intrapelvic tumors after Hemipelvectomy. (f) The serum phosphate level and the clinical course (Used with permission from Ogose et al. [167])
18.6 Malignant Lesions
18.6.1 Synovial Sarcoma
Although synovial sarcoma is a misnomer, as it is not derived from true synovial cells, we will not go into the details of this nosological discussion.
18.6.1.1 Definition
Synovial sarcoma is a clinically and morphologically well-defined entity. It occurs primarily in the para-articular region, usually in close relationship with tendon sheaths, bursas, and joint capsules. It is, however, uncommon in joint cavities. On rare occasions, but widely reported in the radiological literature, it is also encountered in areas without any apparent relationship to synovial structures, such as in the pharynx, the larynx, the tongue, the maxillofacial region, the middle ear, precoccygeal and paravertebral regions, the mediastinum, the thoracic and abdominal wall, the heart, or even in intravascular and intraneural locations [23, 132, 140, 144]. Of note, only synovial sarcoma associated with the locomotor system is discussed in this chapter.
18.6.1.2 Incidence and Clinical Behavior
Epidemiology
Synovial sarcoma accounts for approximately 5–10 % of all malignant mesenchymal neoplasms [90]. It is most prevalent in adolescents and young adults between 15 and 40 years of age (Table 18.1). Men are more susceptible than women with an average ratio of 2:1. There is no predilection for any particular race.
Clinical Behavior and Gross Findings
The most typical presentation is that of a palpable deep-seated soft tissue mass. It is usually associated with pain or tenderness and may cause functional impairment of the adjacent joint. Severe functional disturbances and weight loss are infrequent. Although uncommon, involvement of nearby nerves may cause pain, numbness, or paresthesia.
It is still uncertain whether trauma contributes to the development of synovial sarcoma or not. Although in many patients there is a definite history of trauma, the majority has no such antecedents. When reported, the interval between the episode of trauma and the detection of synovial sarcoma ranges from a few weeks to as much as 40 years. However, there are also reports of patients who sustain injuries after the presence of a mass had been noted, which suggests that, at least in some cases, the relationship between both is purely coincidental.
As mentioned before, synovial sarcoma occurs predominantly in the extremities (80–90 %), mostly near large joints, especially the knee joint (30 %) [45] (Table 18.2). These tumors are intimately related to tendons, tendon sheaths, and bursal structures, usually beyond the confines of the joint capsule. In less than 5 % of all cases, synovial sarcoma arises in the joint space itself.
18.6.1.3 Pathology
The name of the lesion is derived from the microscopic resemblance of synovial sarcoma to normal synovium, although its origin is probably from undifferentiated mesenchymal tissue [45, 115]. Unlike most other types of sarcomas, the tumor is composed of two morphologically different types of cells that form a characteristic biphasic pattern: epithelial cells, which resemble those of carcinoma, and fibrosarcoma-like spindle cells. There are transitions between both types of cells, suggesting a close generic relationship. Depending on the relative presence of these types of cells and on their differentiation, synovial sarcomas can be classified into four different types.
The first type is the biphasic synovial sarcoma that is characterized by the coexistence of morphologically different but histogenetically related epithelial and spindle cells. Calcification with or without ossification is present in about 30 % of tumors. The degree of vascularity varies from a few scattered vascular structures to numerous dilated vascular spaces. In less well-differentiated tumors, hemorrhage may be prominent.
Age range | No. of cases | Percentage (%) |
|---|---|---|
0–10 years | 12 | 3 |
10–20 years | 94 | 27 |
20–30 years | 85 | 25 |
30–40 years | 57 | 17 |
40–50 years | 52 | 15 |
50–60 years | 25 | 7 |
60–70 years | 11 | 3 |
70–80 years | 6 | 2 |
80–90 years | 3 | 1 |
Total | 345 | 100 |
No. of cases | Percentage (%) | ||
|---|---|---|---|
Head–neck | 31 | 9 | |
Neck | 12 | 3 | |
Pharynx | 7 | 2 | |
Larynx | 7 | 2 | |
Other | 5 | 1 | |
Trunk | 28 | 8 | |
Chest | 10 | 3 | |
Abdominal wall | 9 | 2.5 | |
Other | 9 | 2.5 | |
Upper extremities | 80 | 23 | |
Shoulder | 22 | 6 | |
Elbow/upper arm | 20 | 6 | |
Forearm/wrist | 24 | 7 | |
Hand | 14 | 4 | |
Lower extremities | 206 | 60 | |
Hip–groin | 22 | 6 | |
Thigh/knee | 102 | 30 | |
Lower leg/ankle | 33 | 10 | |
Foot | 45 | 13 | |
Other | 4 | 1 | |
Total | 345 | 345 | 100 |
The second type is the monophasic fibrous synovial sarcoma with a predominant or even exclusive spindle cell pattern. This type is relatively common. Its clinical presentation is identical to the biphasic type.
The third type of synovial sarcoma is the monophasic epithelial form. This is a rarely recognized neoplasm with a predominant or exclusive epithelial cell pattern. It is difficult to render the diagnosis of this tumor with certainty since it closely resembles other more frequently occurring epithelial and mesenchymal tumors, such as metastatic and adnexal carcinoma, malignant melanoma, malignant epithelioid schwannoma, and epithelioid sarcoma.
The final type of synovial sarcoma that is histologically recognized is the poorly differentiated type. Its incidence is estimated at 20 %. It behaves more aggressively and metastasizes in a greater percentage of cases. Microscopically, this tumor is composed of small oval or spindle-shaped cells, intermediate in appearance between epithelial and spindle cells. It has a rich vascular pattern with dilated vascular spaces.
Multicystic formation is a common occurrence in synovial sarcoma. The cyst wall is thickened and composed of fibrous septa. Within the cyst, there are often several internal septa, consisting of tumor cells proliferating over the dense collagenous fibrous tissue without a lining pattern. Tumor cells may become detached from the septa in the internal lumen. Furthermore, dilated hemangiopericytomatous vasculatures can be prominent within those septa. Chromosomal rearrangements have been reported in association with synovial sarcoma, consisting of t(X;18) (p11–2;q11–2) translocation [31].
18.6.1.4 Imaging
The majority of synovial sarcomas present on radiographs as round or oval, lobulated masses. They are usually located close to a large joint, particularly the knee joint. In 5–30 % of cases, there is periosteal reaction, bone erosion (related to pressure from the adjacent tumor), or even bone invasion. The most characteristic finding is the presence of multiple small densities caused by focal calcifications or ossifications. This feature is seen in about 20–30 % of cases. It may range from very fine stippling to marked calcifications or even bone formation, typically in the periphery of the lesion. When present, these opacities differentiate synovial sarcoma from liposarcomas and myxoid chondrosarcomas. The irregular shape of the calcifications helps to make the differentiation from hemangioma. In some cases, extensive ossification is present, resembling osseous or cartilaginous lesions such as soft tissue chondroma, extra-articular synovial chondromatosis, ossifying myositis, tumoral calcinosis, or osteosarcoma, both parosteal and extraskeletal. Cases with extensive calcification have been reported to have a better prognosis, with higher survival rates [117, 133].
Angiography usually reveals a prominent vascularity, not only of the primary tumor but also of the metastases. This is especially true for the monophasic and poorly differentiated type of tumor [102]. There is extensive neovascularity and nonhomogeneous staining. Occasionally, the lesion is hypovascular.
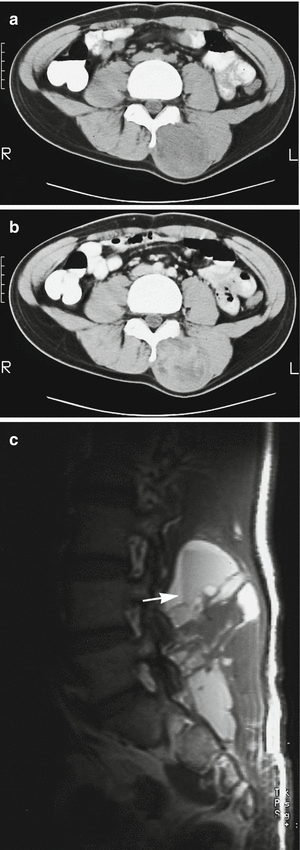
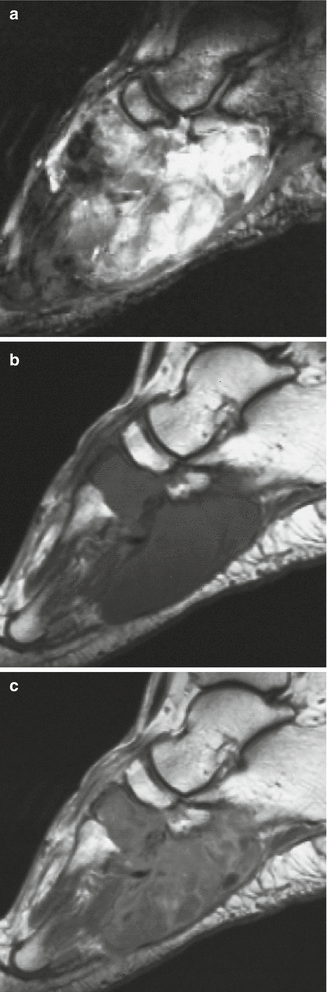
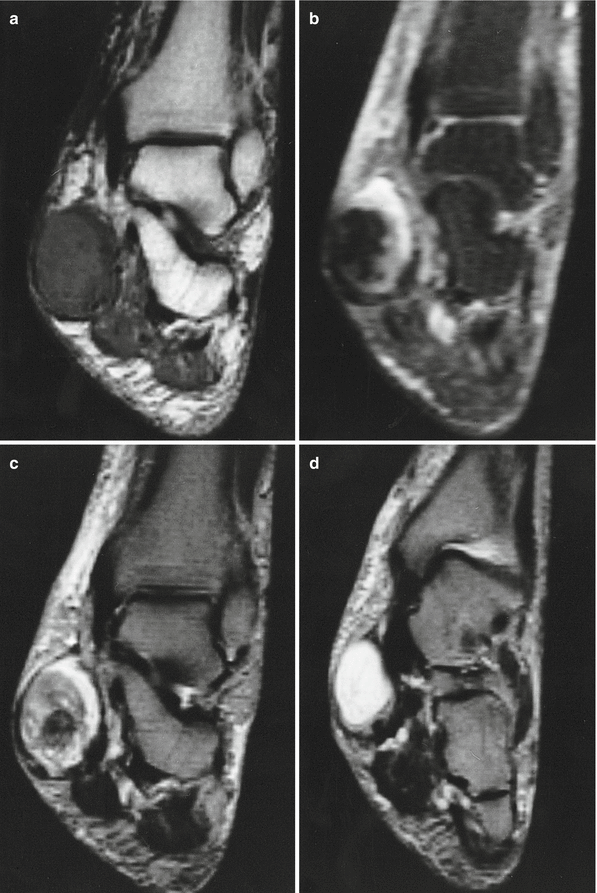

Fig. 18.20
Synovial sarcoma of the back in a 19-year-old man. (a) CT. (b) CT after iodinated contrast injection. (c) Sagittal spin-echo T1-weighted MR image. This is a case of a soft tissue mass in the posterior paravertebral region. There is no clear intratumoral calcification or ossification (a), and only mild enhancement is noted after injection of iodinate contrast medium (b). Fluid–fluid levels can sometimes be seen on the T1-weighted image (c), but are a rare and nonspecific sign (arrow)

Fig. 18.21
Synovial sarcoma of the foot in a 37-year-old man. (a) Sagittal gradient-recalled echo T2*-weighted MR image. (b) Sagittal spin-echo T1-weighted MR image. (c) Sagittal spin-echo T1-weighted MR image after gadolinium contrast injection. Synovial sarcoma is generally hypointense on a T1-weighted image (b) and hyperintense on a T2-weighted image (a). Large lesions such as this one are usually heterogeneous and may show internal septation (a). Marked enhancement is correlated to the extensive vascular supply of these tumors (c). Bone invasion is less frequent (5–30 %), but can be quite important. The sole of the foot is a common localization of synovial sarcoma in young adults

Fig. 18.22
Synovial sarcoma at the medial side of the ankle. (a) Coronal T1-weighted MR image. (b) Contrast-enhanced coronal fat-suppressed T1-weighted MR image. (c, d) Coronal fat-suppressed T2-weighted MR images. Oval mass, medially at the ankle joint, with inhomogeneous low signal intensity (a). Strong contrast uptake is seen at the periphery of the lesion (b). A triple signal pattern is seen within the lesion with signal intensities similar to fluid, intermediate, and low signal intensities (c, d)
Ultrasound characteristics are those of a well-defined, solid vascular tumor with prominent arterial and venous components. There may be internal cystic components due to internal hemorrhage [107]. Internal calcifications are noted in 20–30 %. Ultrasound appears useful as a detection method of local recurrence, in the guidance of fine-needle biopsy and in the examination of children [72]. In the early postoperative period (less than 6 months after surgery), inhomogeneous hypoechogenic lesions cannot be differentiated with certainty as recurrent tumor, hemorrhage, edema, granulation tissue, abscess formation, or any combination of these. Performed with high-frequency transducers, a lesion is considered to be a recurrent tumor when a discrete nodular hypoechogenic mass is present. Areas of diffuse abnormal echogenicity without evidence of a discrete nodule can be classified as non-tumoral.
CT shows a soft tissue mass, which may infiltrate adjacent structures, having a slightly higher density than muscle [115] (Fig. 18.20). Joint invasion is present when the soft tissue mass projects into the expected confines of a joint capsule or when an intra-articular ligament or tendon is involved. Although bony involvement can be identified on both MR and CT imaging, cortical bone erosion or invasion is better depicted on CT. Intratumoral calcification or ossification is also more easily seen on CT than on MR imaging. Because of its extensive vascular supply, synovial sarcoma enhances markedly after injection of contrast medium.
On MR imaging, most synovial sarcomas (>90 %) are hypointense relative to fat and nearly isointense relative to muscle on T1-weighted MR images [117]. In rare cases, the tumor may be mostly hyperintense due to extensive intratumoral hemorrhage. Small areas of high signal on T1-weighted images are more often encountered (45 %) [81, 117, 123]. They probably correspond with small foci of hemorrhage, since these areas are of high signal on T2-weighted images, too. Fluid–fluid levels, although not very frequent (15–25 %) and not specific, can be a striking finding. Areas of previous hemorrhage with fluid–fluid levels or high signal intensity on all pulse sequences may be associated with a worse prognosis [117] (Figs. 23.13–23.17). On T2-weighted images, marked inhomogeneity is the rule, and various degrees of internal septation may be noted [114] (Figs. 18.21 and 18.24). This is especially true for lesions more than 5 cm in diameter which show this heterogeneous signal pattern in more than 85 % of cases vs. 60 % for smaller lesions [81]. A triple signal pattern on T2-weighted images is described in one third of all synovial sarcomas [12, 81, 133]. It consists of high signal similar to fluid, intermediate signal intensity equal to or slightly hyperintense relative to fat, and low signal intensity closer to that of fibrous tissue. This triple signal pattern on T2-weighted images together with the small high signal foci on T1-weighted images is suggestive for synovial sarcoma (Figs. 18.22 and 18.23). More than half of all lesions are intimately related to bone. There seems to be no notable difference between the MR imaging characteristics of the mono- and biphasic pathologic subtypes.
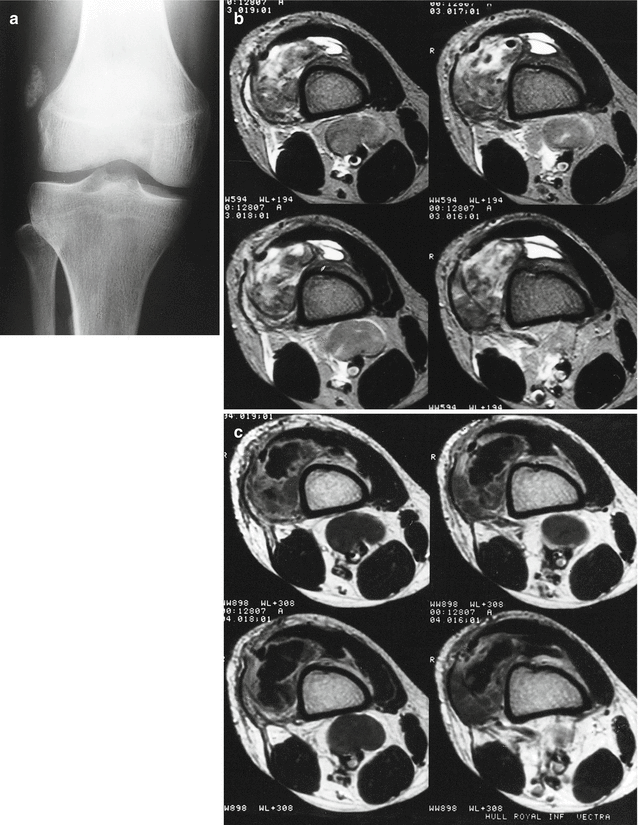

Fig. 18.23
Synovial sarcoma of the knee. (a) Anteroposterior radiograph of the right knee. (b) Axial T2-weighted MR image at the distal femur. (c) Axial T1-weighted MR image after intravenous gadolinium administration. A crumbly calcification is seen within a palpable soft tissue mass at the lateral side of the knee. Calcifications are seen in up to 30 % of synovial sarcomas (a). The tumor is of mixed signal intensity with hypointense and hyperintense areas, as well as components of intermediate signal intensity (“triple signal”); the tumor looks to be arising outside the joint, but extends down the lateral patellar retinaculum into the superolateral aspect of the knee joint (b). There is marked peripheral contrast enhancement within the mass, with a central irregularly delineated area of absent enhancement. There are enlarged lymph nodes around the neurovascular bundle in the popliteal fossa (c) (Case courtesy of Dr. A.M. Davies, Birmingham)
Heterogeneous enhancement after injection of contrast material is generally seen. Hypervascular areas are strongly enhancing, whereas necrotic and cystic areas remain hypointense. Hypervascularity may be quantified by a dynamic contrast-enhanced MR examination. Early enhancement of tumor within 7 s after arterial enhancement is a reliable sign, occurring consistently in synovial sarcoma. Other previously described so-called malignant dynamic contrast-enhanced MR imaging features, such as early plateau or washout phase and peripheral enhancement are not always found in synovial sarcoma [155].
Well-defined or reasonably well-defined margins are seen in some cases of synovial sarcoma. This finding has been described as a probable sign of benignity [132, 133]. This may be one of the reasons why synovial sarcoma is the malignant tumor most frequently misdiagnosed as benign [17, 133]. Neurovascular involvement may be suspected in cases where the tumor margin abuts and displaces the neurovascular bundle. In one study, this was confirmed at operation in two out of five cases [114]. MR often fails to demonstrate small calcifications seen on plain films or CT. Therefore, the MR images of soft tissue and bone tumors should be interpreted with corresponding CT images and plain films.
The differential diagnosis on MR imaging of an inhomogeneous septate mass with infiltrative margins and located in close proximity to a joint, a tendon, or a bursa is very limited. Without the history of trauma or signs of infection, a malignant neoplasm is the most likely consideration. Especially in combination with soft tissue calcifications, the diagnosis of a synovial sarcoma should be preferred. However, a septate configuration can also be seen in two types of benign soft tissue masses: hemangioma and synovial or ganglion cysts. Typically, these benign tumors are sharply marginated and have a homogeneous hyperintense signal that is much brighter than that of the subcutaneous fat on T2-weighted images. The pattern of contrast enhancement may be helpful as well in the differential diagnosis (Figs. 18.24 and 18.25).
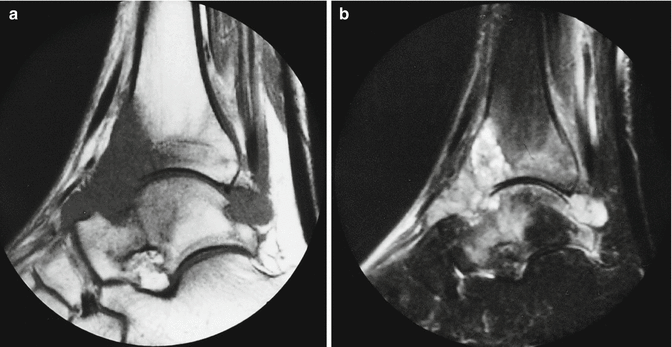
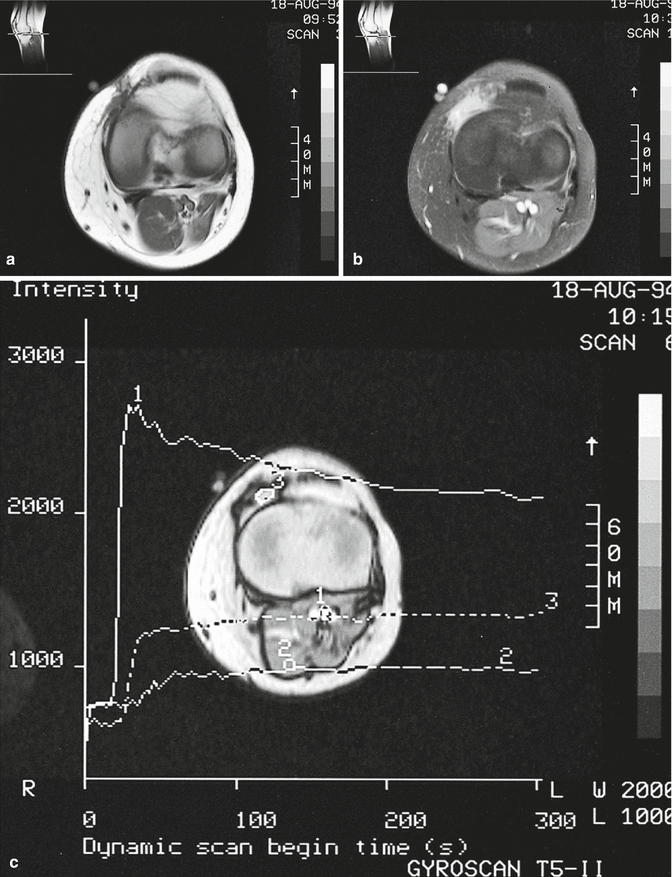

Fig. 18.24
Synovial sarcoma developing within the ankle joint. (a) Sagittal T1-weighted MR image. (b) Sagittal STIR image. A low signal intensity mass is present within the anterior and posterior recess of the ankle joint. There is associated erosion of the talar neck. The anterior cortex of the distal tibia is destroyed. Bone marrow edema is seen within the distal tibia and in the talus (a). The tumor is of high signal intensity on T2-weighted images, with some internal low signal intensity septa. The adjacent bone invasion and bone marrow edema are better appreciated than on the T1-weighted images (b) (Case courtesy of Dr. A.M. Davies, Birmingham)

Fig. 18.25
Recurrent synovial sarcoma. (a) Axial spin-echo T1-weighted MR image. (b) Axial, fat-suppressed, spin-echo T1-weighted MR image after gadolinium contrast injection. (c) Signal intensity-versus-time curve calculated on a gradient-recalled echo T1-weighted MR image during gadolinium contrast injection. Synovial sarcoma quite frequently recurs after surgical excision. In this case, a small hypointense lesion located deep in the scar tissue can be noted on the T1-weighted image (a). After gadolinium injection, clear enhancement of this region can be noted on the fat-suppressed images (b). Dynamic imaging during bolus contrast injection (c) demonstrates the higher and more rapid uptake of contrast medium by this tissue (3) than by normal muscle (2). Repeat surgery disclosed recurrent tumor
18.6.1.5 Treatment, Prognosis, and Detection of Tumor Recurrence
Treatment of synovial sarcoma consists of wide local excision or limb amputation, usually in combination with chemotherapy and radiation therapy. Despite this aggressive therapy, metastatic disease or local recurrence is found in approximately 80 % of patients. Metastases most frequently affect the lung (59–94 % of distant tumor spread). Additional sites of metastatic disease include lymph nodes (4–18 %) and the bone (8–11 %). Soft tissue metastases are rare. Local recurrence occurs in 20–26 % of patients and presents usually within 2 years after initial diagnosis. The 5-year survival rate is approximately 27–55 %. Favorable prognostic factors with synovial sarcoma include extensive calcification, younger patient age, lesions less than 5 cm in diameter, and neoplasms located in the extremities [117]. According to Tateishi, other prognostic MRI parameters include the presence of hemorrhage and cysts or the presence of a triple signal. Together with proximal tumor distribution, large tumor size, and the absence of calcification, these parameters are associated with high tumor grade and a less favorable prognosis [151]. The value of MRI in the early detection of tumor recurrence will be discussed in detail in Chap. 26, dealing with imaging posttreatment (Fig. 18.25).
18.6.2 Epithelioid Sarcoma
18.6.2.1 Definition
The term “epithelioid sarcoma” was applied by Enzinger in 1970 to refer to a distinctive neoplasm that commonly involves the soft tissues of the extremities, most frequently of the hand. This tumor presents as a solitary or irregular multinodular mass located in the dermis or deep-seated and attached to tendons or fascia. The size of the lesion is variable and ranges from several millimeters to more than 15 cm in diameter. Central degeneration and necrosis are common features. Lesions located in the dermis often ulcerate through the skin. The tumor may spread along the neurovascular bundles, may invade the vessels, and, hence, may metastasize. In general, tumors located in the proximal extremity reveal a more aggressive course than those arising in the distal extremities [66]. A history of trauma is reported in up to 25 % [25, 129]; histogenesis, and the nature of this neoplasm remains unknown.
18.6.2.2 Incidence and Clinical Behavior
Epithelioid sarcoma represents the most common soft tissue sarcoma of the hand. Although it may occur at any age, the tumor is found most commonly in adolescents and young adults between 10 and 35 years of age. Children and older persons are only rarely affected. This tumor is twice as common in men as in women. The principal sites of involvement are the distal upper extremity, involved in 58 % of cases, particularly the hands and forearms. This main site of involvement is followed by the distal and proximal extremity, at 15 and 12 %, respectively. The trunk, head, and neck regions, with the exception of the scalp, are seldom involved [66]. Lesions may be located in the subcutis or are deep seated, usually attached to tendons, tendon sheaths, or fascial structures. Epithelioid sarcoma presents as a firm, hard nodule that may be solitary or multiple. These nodules are slowly growing and painless. Ulceration through the skin is common in intradermal lesions. Metastases, predominantly to the regional lymph nodes and lung, are observed in less than half of the cases. These develop mostly within the first year after diagnosis, but may be late and become apparent for many years after excision of the primary tumor. Recurrences, often multiple, are common and have been reported in 77 % of cases [25].
18.6.2.3 Imaging
Imaging Studies Other than MRI
MRI Findings
No characteristic MRI features of epithelioid sarcoma exist [66]. Hence, MRI does not enable a specific diagnosis of this tumor. On T1-weighted images, the tumor appears mostly homogeneous and is isointense with muscle (Fig. 18.26). Occasionally, lesions may be heterogeneous, with either areas of increased signal, due to foci of hemorrhagic necrosis, or with relative central hypointensity, corresponding to extensive intratumoral necrosis. In contrast, on T2-weighted images, the tumor is hyperintense to the muscle, hypo-, iso-, or hyperintense to fatty tissue. A majority of lesions is homogeneous on these T2-weighted sequences. Peritumoral edema affecting the surrounding muscles is observed in nearly 70 % of cases. It typically appears as a high signal intensity area on T2-weighted or STIR images, with variable shape, or it may present as a feathery, radial pattern of increased signal intensity, extending for a maximum of 2 cm into the adjacent muscle, or as an extensive area of abnormal signal, involving completely at least one muscle [66]. Other common findings include tumor encasement of the adjacent neurovascular bundle and enlarged regional lymph nodes. The latter often shows the same increased signal intensity on T2-weighted images as the primary tumor. Inhomogeneous but strong enhancement is observed following administration of gadolinium chelates, except at the areas of central necrosis [66].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree