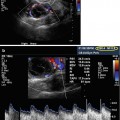
Fig. 23.1
Ovary with normal antral follicle count
Several investigators have evaluated AFC to predict the development of OHSS. Kwee et al. evaluated 110 patients with unexplained infertility, male factor, or cervical factor infertility, counted antral follicles in all patients, and correlated the number of antral follicles with level of ovarian response to IVF [2]. They categorized ovarian response as poor, normal, and high and then calculated the AFC cutoff which most accurately identified each group. The AFC value of >14 identified hyper-responders with a sensitivity of 82 % and a specificity of 89 %. Oncal et al. also evaluated the predictive role of AFC in OHSS in 41 women identified to have moderate to severe OHSS and 41 age-matched controls who did not develop OHSS [3]. They found that AFC had a moderate accuracy to predict the development of OHSS. Using an AFC cutoff of eight, much lower than the AFC cutoff used by Kwee et al., they calculated 78 % sensitivity and 65 % specificity. In a meta-analysis done by Broer et al. investigating AFC as a predictor of ovarian hyperstimulation, five studies were identified which met criteria for inclusion [4]. Two reported on AFC alone, three reported on both anti-Mullerian hormone (AMH) and AFC, and all five were prospective cohort studies. Among the included studies, the definition of excessive ovarian response to IVF varied between ≥15 and ≥20 oocytes. Importantly, the number of oocytes retrieved was used as a surrogate for risk of OHSS; no study specifically identified the patients who met diagnostic criteria for OHSS. When these five studies of AFC were evaluated together, the sensitivity of AFC to predict ovarian hyper-response was seen to vary between 20 and 94 % depending on the AFC cutoff used, and the specificity varied between 33 and 98 %. From these values, the authors calculated a sum estimate of the sensitivity to be 82 % and a sum estimate of the specificity to be 80 %. Considering this evidence, there is a clear association between increased AFC and increased risk of OHSS. Given the sensitivity and specificity estimates of AFC in the studies to date, if the AFC is found to be greater than 14–16, caution should be executed in the initial gonadotropin dosing and choice of stimulation protocol since there is clearly increased risk of ovarian hyper-response in such patients.
When proposing the use of AFC to predict OHSS, it is important to be aware of the variability both in definition of antral follicle and operator technique in follicle counting [5]. Interestingly, some authors adhere to the definition of antral follicle as those follicles which are measured to be 2–10 mm in the early follicular phase, as above; but other authors limit that definition to only those follicles which measure 2–5 mm. The precise menstrual timing of the measurement of AFC is also important. AFC should be performed either between cycle day 2 and 4 or while on oral contraceptive pills for greatest accuracy and reproducibility.
The number of growing follicles in response to gonadotropin stimulation during ART is another sonographic test which has been proposed to predict the development of OHSS. Clearly, there is a connection between the ovarian response to stimulation and AFC, as patients with higher baseline AFC would be expected to have a more robust ovarian response to treatment. Papanikolaou et al. sought to correlate the number of follicles ≥11 mm growing in response to gonadotropin treatment during IVF with the likelihood of developing moderate or severe ovarian hyperstimulation syndrome [6]. They evaluated 1,801 patients undergoing IVF treatment over a 2-year period. Factors such as peak estradiol level and number of follicles ≥11 mm were correlated with the development of OHSS. In this cohort, 53 patients were hospitalized because of OHSS. They found that a threshold of ≥13 follicles measuring ≥11 mm was predictive of the development of OHSS with a sensitivity of 85.5 % and a specificity of 69 %. Interestingly, the number of follicles ≥11 mm was a much better predictor of OHSS than the peak serum estradiol level, which had only a 53 % sensitivity and 77 % specificity. Therefore, if it becomes apparent during an IVF cycle that there are 13 or more follicles measuring ≥11 mm, the patient and physician should both be cognizant of the increased risk of developing OHSS regardless of the serum estradiol level.
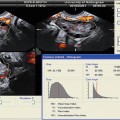
Because patients who have higher baseline AFC and higher functional ovarian response to stimulation have been found to have a greater likelihood of developing OHSS, it is important to identify such patients early in clinical care. It is well known that patients with polycystic ovarian syndrome (PCOS) have by definition a high antral follicle count and magnified response to IVF. Ultrasound assessment of ovarian morphology serves as one of the key criteria for the diagnosis of PCOS by the Rotterdam criteria [7]. The sonographic findings which meet Rotterdam diagnostic criteria are either 12 or more follicles in each ovary measuring 2–9 mm in diameter and/or increased ovarian volume >10 mL [8] (Fig. 23.2). In combination with either anovulation/oligo-ovulation or clinical/biochemical hyperandrogenism and having excluded other endocrine conditions such as Cushing’s syndrome and congenital adrenal hyperplasia, a patient could be diagnosed as having PCOS. Interestingly, even women who do not technically meet criteria for PCOS but have isolated polycystic-appearing ovaries on ultrasound have been found to have a higher risk of developing OHSS [7]. Therefore, clinical management including gonadotropin dosing and choice of stimulation protocol should incorporate knowledge of PCOS or polycystic-appearing ovaries on ultrasound in an attempt to minimize the development of OHSS in these patients.
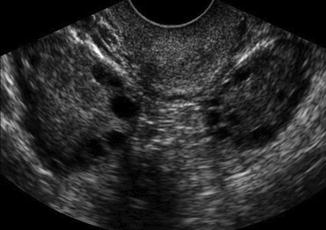

Fig. 23.2
AFC of PCOS ovary in 2D ultrasound
Using patients with PCOS as a model for other patients at risk for OHSS, researchers have investigated ultrasound calculation of baseline ovarian volume alone as a predictive marker. Danninger et al. studied 101 patients undergoing IVF, all of whom had 3D volumetric assessment of ovarian volume starting on stimulation day 1 [9]. The authors then remeasured ovarian volume on the day of human chorionic gonadotropin (hCG) and correlated those findings with the development of OHSS. They found a significant correlation between the baseline ovarian volume and OHSS (p = 0.03) with a greater baseline ovarian volume in women who subsequently developed OHSS compared to those who did not. The authors estimated an ovarian volume cutoff of 10 mL as predictive of OHSS. Importantly, this sonographic finding was not as robust as some of the other markers already discussed. Even in the 34 patients identified to have an ovarian volume >10 mL, only 23.5 % ultimately developed OHSS. Although it is logical in the context of PCOS and the PCOS-associated risk of OHSS, the measurement of ovarian volume is not considered to be a standard marker at this time to predict OHSS.
The final ultrasound characteristics which have been used to attempt to predict the development of OHSS are Doppler flow studies of ovarian vasculature. The concept behind the assessment of ovarian vascular resistance and flow is that because OHSS involves third spacing of fluid secondary to increased vascular permeability, one might expect changes in ovarian vascular flow which may occur prior to clinical signs or symptoms of OHSS and therefore could be used to predict the development of OHSS [10]. Coupled with increased vascular permeability, there is also abnormal intraovarian angiogenesis in OHSS leading to low vascular impedance. Multiple authors have investigated sonographic characterization of ovarian vascular flow, resistance, peak systolic velocity, and pulse-wave power Doppler to try to correlate vascular chances with the likelihood of developing OHSS. In 1997, Moohan et al. evaluated 30 patients who were diagnosed with mild or severe OHSS within 2–15 days of oocyte retrieval [10]. All patients underwent transabdominal ultrasound at the time of diagnosis of OHSS with color Doppler done on low-flow setting to characterize the flow velocity waveforms within the ovarian vessels. Vascular pulsatility index, resistance index, S-D ratio, and maximal peak systolic velocity were calculated. The authors found that in patients with severe OHSS, there was markedly reduced vascular impedance with a statistically significantly higher resistance index in patients with mild OHSS compared to severe (0.49 vs. 0.41, p < 0.005). Surprisingly, there was no difference in maximal peak systolic velocity, but pulsatility index and S-D ratio also differed significantly between patients with mild and severe OHSS. Of importance, this study evaluated only patients diagnosed with OHSS. There was no comparison with patients who did not develop OHSS, so it is impossible to know if these differences in vascular flow could have been used to predict the development of OHSS. Other authors including Agrawal et al. did compare patients with OHSS to controls and found a difference in ovarian stromal peak systolic velocity and time-averaged maximal velocity between patients with and without OHSS [11]. In the study by Agrawal et al. published in 1998, ovarian Doppler flow velocity was statistically significantly higher in patients with OHSS than controls, but pulsatility index and resistance index did not differ between the groups. The authors concluded that the changes in flow velocity correlated with changes in vascular endothelial growth factor (VEGF) serum and follicular fluid concentrations. More recently, Jayaprakasan et al. used three-dimensional (3D) power Doppler angiography to attempt to predict OHSS [12]. In 118 patients, of whom 18 developed moderate or severe OHSS, ovarian vascular flow indices were quantified by 3D ultrasound. Unexpectedly, there was no difference in vascularization index, flow index, or vascularization flow index between either patients with OHSS vs. controls or the subgroups of patients with moderate vs. severe OHSS. Therefore, although the pathophysiology of OHSS involves known changes in vascular permeability which logically suggest a connection between Doppler measurements of ovarian vascular flow and the development of OHSS, unfortunately no studies to date have convincingly shown that ultrasound measurement of ovarian vascular parameters can be used to predict the risk of OHSS.
In summary, ultrasound has been investigated as a tool to predict the development of OHSS through assessment of AFC, quantitation of follicular development during IVF, identification of polycystic-appearing ovaries or PCOS, determination of ovarian volume, and Doppler flow studies of ovarian vasculature. Of these potential sonographic markers, AFC is the most significant predictor of the development of OHSS and should be used to guide management. There is also evidence linking the number of developing follicles and the diagnosis of PCOS with the risk of OHSS. To date, the other ultrasound tools including ovarian volume and vascular flow analysis do not have clinical utility in the prediction of OHSS.
Ultrasound in the Diagnosis of Ovarian Hyperstimulation Syndrome
Once OHSS is suspected on clinical grounds, the diagnosis is aided by ultrasound findings. OHSS is categorized into mild, moderate, and severe disease. The differentiation involves sonographic features including the degree of ovarian enlargement, presence and volume of abdominal ascites, presence or absence of pleural effusions, and Doppler studies showing venous thromboembolism [13].
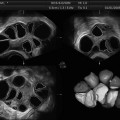
The clinical findings of OHSS encompass a spectrum ranging from mild disease, unpleasant for the patient but not considered to be dangerous, to severe OHSS with significant consequences and risk of death. Mild OHSS is common and involves symptoms such as lower abdominal or pelvic discomfort, gastrointestinal complaints including nausea, emesis, and diarrhea, and some degree of abdominal distention [14]. The process of superovulation frequently leads to these mild manifestations of OHSS, and up to a third of IVF cycles may involve these complaints. The only sonographic characteristic of mild OHSS may be enlarged ovaries (5–12 cm) [15] (Fig. 23.3).
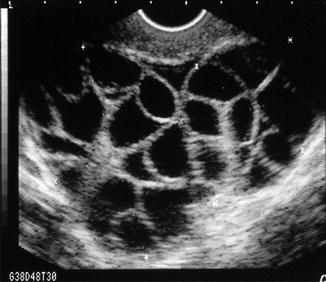

Fig. 23.3
Hyperstimulated ovary after gonadotropin therapy
Moderate OHSS consists of intensified pain, nausea or emesis, enlarged ovaries seen on ultrasound, and sonographic identification of abdominal or pelvic ascites with normal serum laboratory parameters (Fig. 23.4a). Some authors have described OHSS as an abdominal compartment syndrome because the rapid accumulation of ascites can lead to increased intra-abdominal pressure [16]. Increased intra-abdominal pressure can become acute and lead to organ dysfunction. In severe forms, abdominal compartment syndrome affects respiratory function, as in the case of severe OHSS.
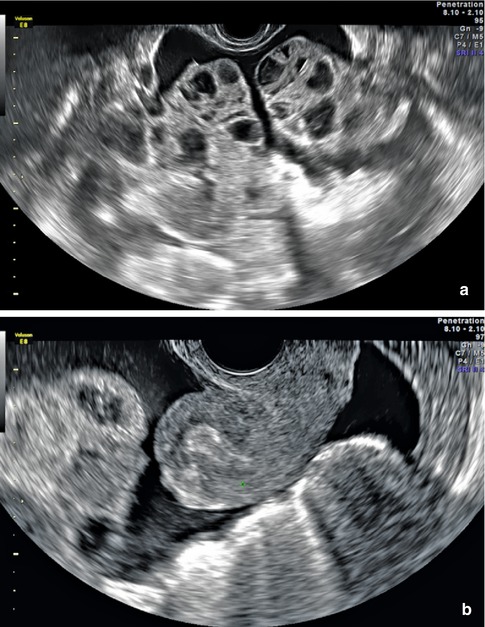

Fig. 23.4
(a) Ultrasound of moderate OHSS with ascites. (b) Ultrasound of ascites in the cul-de-sac with severe OHSS
Given that abdominal ascites is a key characteristic of the diagnosis of moderate OHSS, it is critical that the ultrasound findings be interpreted correctly in the context of the clinical presentation. Gunabushanam et al. reported the case of a 22-year-old woman who had received fertility treatments and presented to the emergency department complaining of a 12 h history of severe lower abdominal pain [17]. Transabdominal ultrasound showed enlarged ovaries bilaterally (7 × 5 × 5 cm) with significant anechoic peritoneal free fluid felt consistent with ascites. She was diagnosed with OHSS. She then began to clinically decompensate with the development of pallor and peritoneal signs and underwent diagnostic paracentesis notable for non-clotting blood. Ultimately she was taken to the operating room for emergent laparotomy and a bleeding ovarian cyst was identified and treated. This case demonstrates the dangers of assuming the diagnosis of OHSS in all patients undergoing fertility treatments, as other pelvic pathology can clearly lead to the accumulation of pelvic fluid. Cyst rupture can certainly lead to significant intraperitoneal bleeding with risk of death, and accurate communication between the sonographer, Radiologist, Emergency Department physician, and Reproductive Endocrinologist is critical to appropriate and timely diagnosis.
Severe OHSS is one of the most serious complications of ovarian hyperstimulation (Fig. 23.4b). The incidence of severe OHSS is estimated between 0.5 and 5 % per IVF cycle. Severe OHSS has been reported to be fatal, so the prompt diagnosis and treatment is paramount [18]. Patients with severe OHSS describe rapid weight gain, significant abdominal distention with inability to fit into usual clothes, shortness of breath, pain which can be refractory to oral medications, oliguria, and severe and unrelenting nausea or emesis with inability to tolerate oral intake. Clinical findings include all of the features of moderate OHSS plus clinical ascites, sonographic ascites, ultrasound evidence of pleural effusions, and serum laboratory abnormalities such as hemoconcentration, coagulopathy, electrolyte imbalance, and renal and hepatic dysfunction or failure [19].
Current research indicates that the fluid shifts which occur in OHSS are directly caused by increased VEGF. VEGF leads to increased vascular permeability, reduced colloid osmotic gradient, and spillage of fluid out of the vascular compartment and into the extravascular spaces [20]. These fluid shifts can be identified sonographically, and it is recommended that in the evaluation of the patient suspected to have moderate or severe OHSS, ultrasound should be used to check for abdominal ascites or pleural effusions. Generally, the volume of accumulated fluid is not subtle and can easily be identified either through abdominal or vaginal ultrasound or ultrasound of the lung bases. The third spacing of fluid into the peritoneal and pleural cavities leads to respiratory compromise, hypotension, increased intra-abdominal pressure, and renal compromise related to decreased perfusion [21].
The hemoconcentration and resultant hypercoagulability of severe OHSS can lead to venous and arterial thromboembolism both in the typical locations such as lower extremities and lungs and in sites which seem more specific to OHSS such as the subclavian and internal jugular vessels. It is unclear why thrombosis may be localized to the neck rather than the lower extremities; some have hypothesized that increased peritoneal fluid containing inflammatory mediators drains into the thoracic duct and directly into the subclavian veins, possibly locally increasing coagulation at those sites [22]. Rova et al. evaluated the risk of venous thromboembolism in all IVF cycles and particularly in the subset complicated by OHSS [21




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree