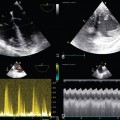
10 Central venous access is a core skill for anesthesiologists, intensivists, acute care physicians, and surgeons. It is indicated for fluid and drug administration, dialysis, and cardiovascular monitoring. More than 5 million central venous catheters (CVCs) are inserted annually in the United States.1 Because central venous access often remains poorly taught and left out of training curricula, inexperienced and less skilled practitioners frequently experience significant difficulties. Ultrasound guidance (USG) for CVC placement was introduced in the 1980s. Since then, many reports have demonstrated increased success, enhanced safety, and effectiveness of USG in comparison to anatomic landmark or audio Doppler-based techniques.2–5 Several national and international recommendations6–10 have been developed that advocate the routine use of USG for placement of CVCs. Although the original practice guidelines11 focused mainly on USG for cannulation of the internal jugular vein (IJV), USG can be used at most sites. To appreciate the importance of ultrasound-guided vascular access (USGVA) it is necessary to understand the potential complications of attempted vascular access, which can be grouped under immediate and delayed complications. The former include inadvertent arterial or venous puncture and cannulation, hematoma, posterior vessel wall (through-and-through) puncture, pneumothorax or hemothorax, and air embolism. Other complications include multiple skin punctures, patient anxiety and discomfort, and failure of the procedure. Thoracic duct injury and nerve injury may follow CVC placement. Late complications of vascular access are local and distant infection, including catheter-related bloodstream infection (CRBSI), arterial or venous thrombosis, vessel stenosis, occlusion of the lumen or device, arteriovenous fistula formation, and tip migration.1,6 USGVA can potentially reduce the incidence or completely prevent many of these complications and improve the safety of the procedure.2–5 This is due to a reduction or avoidance of primary damage caused by injury to collateral structures from the needle or secondary damage caused by misplaced guidewires, dilators, and catheters. USG enables direct visualization of the vessel or vessels of interest and surrounding structures. The depth and size of the target vessel can be measured and an optimal target vessel and site for venipuncture chosen. In trained hands, USGVA reduces the frequency of unsuccessful punctures and associated complications and facilitates first-pass cannulation.2–5 A preprocedural scan is performed to identify abnormal anatomy, thrombosis, or valves, and a postcannulation scan is used to confirm correct placement of the guidewire and catheter in the intended vessel. Ultrasound can be used reliably for the diagnosis of pneumothorax or pleural fluid collections. The same transducer used for cannulation can be used to examine the pleura.6 There is a potential reduction in CRBSI when USGVA is integrated within a multifaceted strategy.3 USGVA is cost-effective because of better clinical outcomes and reduced cost of care.2,6,11 Application of electric current to the piezoelectric element in the transducer creates ultrasound waves (see Chapter 1). The mechanical energy generated travels and penetrates through the tissues as pulsed longitudinal waves, some of which will be absorbed or lost in the tissues whereas the remainder will be reflected back at different tissue interfaces and received at the transducer. The phenomenon of attenuation describes the loss of ultrasound energy via absorption, reflection, refraction, and scattering and can cause distortion of the image and misinterpretation of anatomic relationship. The returned energy is processed into an anatomic representation of the underlying structures (see Chapter 1).12 Broad-frequency transducers (5 to 15 MHz) are typically used for vascular access. Higher frequencies provide better resolution, are best suited for superficial structures, and enable identification and avoidance of adjacent vulnerable structures (e.g., small arteries and nerves). High resolution is also required for neonatal and pediatric cannulation.9,12 Midrange frequencies (5 to 10 MHz) are used to visualize structures 3 to 6 cm under the surface of the skin. A low-frequency probe operates below 5 MHz and is usually reserved for deeper structures; it is not commonly used for vascular access.6,9 The scanning surface (footprint) may be linear or curvilinear. Typical footprints vary from 20 to 50 mm in length. Smaller footprints enable access to confined anatomic locations (e.g., infraclavicular imaging or children), whereas large footprints allow the acquisition of a wider image.6,9 Safe, successful vascular access requires attention to the surrounding environment: ultrasound display position and transducer orientation. Operator experience and patient and equipment factors contribute to the final outcome. Adequate knowledge of the physics of USG and the mechanism of image acquisition and interpretation is essential before attempting USGVA. The operator must have the ability to interpret the displayed two-dimensional (2D) image of the vessels of interest and associated tissues, use this 2D image to perform a three-dimensional (3D) task, and possess the necessary hand-eye coordination and manual dexterity skills to manipulate the needle and transducer according to the displayed image.9 The display should be positioned at an appropriate height for the procedure and on the contralateral side of the patient.6,9 The displayed image should be in the same anatomic orientation as seen from the position of the practitioner. A palpable marker on the side of the transducer corresponds to an orientation cue on the display. Touching one end of the probe and observing the associated display artifact will eliminate confusion.12 Typically, the operator holds the ultrasound probe with the nondominant hand in such a way that the structures beneath the left end of the probe appears on the left side of the screen and vice versa, and subsequently each side of the screen will display the ipsilateral structures.9,12 Depth and gain control is used to improve the quality of the displayed image.12 This typically follows a preliminary scan and identification of the structures of interest. Initially, the depth must be adequate to allow visualization of a wider anatomic field that includes the structure or structures of interest. This will identify vulnerable structures and those at risk with needle advancement, for example, the pleura and other vessels. After a preliminary scan, the depth can be reduced to focus on the target vessel and associated structures. Sometimes the operator may want to increase or decrease the gray scale for optimization of the image. The gain control is used to reduce or amplify the ultrasound signals received and subsequently image brightness.12 Different structures have different echogenic characteristics, and this will influence the amount of gain control required. Relative echogenicity refers to the ability of some structures to reflect back more of the emitted ultrasound energy. Hyperechoic or echogenic structures (e.g., bones and pleura) reflect more ultrasound signal than do the surrounding tissues and appear brighter. In contrast, blood and fluid are hypoechoic.12 Ultrasound-assisted vascular access refers to the use of ultrasound to verify the presence and patency of vessels whose approximate position can then be marked on the skin. This is followed by an attempted “blind” vascular puncture.6 Ultrasound-guided cannulation refers to ultrasound scanning to visualize and verify a vessel and subsequently guide the tip of the needle in real time throughout the insertion process.6 Vascular structures have different morphologic and anatomic features. Ultrasound examination is used to distinguish veins and arteries and avoid unintentional puncture or cannulation. Veins are typically elliptic or ovoid, thin walled, and readily collapsible, whereas arteries are circular, thick walled, and less compressible.9 Though not routinely required for vascular access, Doppler examination can demonstrate vessel patency and the direction and nature of blood flow and enable differentiation of deeper veins from arteries. Color Doppler and spectral Doppler waveform examination require further training and can establish vessel patency, as well as differentiate between a vascular and a nonvascular hypoechoic structure.6,9
Ultrasound-guided central venous access: The basics
Overview
Advantages of ultrasound-guided vascular access
The ultrasound transducer
The display
Image optimization
Vascular scanning
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine