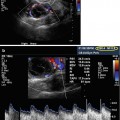
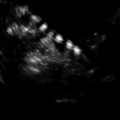
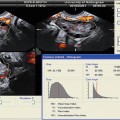
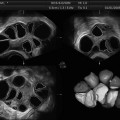
Fig. 21.1
Ultrasound guidance at time of hysteroscopy (Black arrow pointing at hysteroscope, white arrow pointing at hysteroscopic scissors)
Uterine Septum
The incidence of congenital uterine anomalies is estimated to be 3–4 % of reproductive age women and 5–10 % in women with recurrent miscarriages [5, 6]. The anomalies may be asymptomatic or result in infertility, recurrent pregnancy loss, intrauterine growth retardation, or endometriosis when obstruction is present. Uterine septum, which results from incomplete resorption of the medial septum between two normally fused hemi-uteri, is not only the most common uterine anomaly but also is the malformation most highly associated with poor pregnancy outcomes [5, 7]. Case series report a spontaneous miscarriage rate ranging from 65 to 88 % in the presence of a uterine septum [8, 9]. It is hypothesized that implantation on a poorly vascularized septum may contribute to this increased risk of miscarriage. After resection, spontaneous miscarriage rates return to the baseline rate of approximately 15 %; 80 % of pregnancies post-resection result in term delivery [7, 8].
The high degree of accuracy of magnetic resonance imaging (MRI) and three-dimensional (3D) ultrasound in the diagnosis of congenital anomalies has decreased the previous need for diagnostic laparoscopy at the time of surgery to aid in characterization of the type of anomaly. Three-dimensional ultrasound has been shown to have 98 % specificity and 100 % sensitivity of accurate septum diagnosis [10]. Although diagnostic hysteroscopy/laparoscopy is still considered the gold standard, MRI and 3D ultrasound are accepted alternatives with both lower risk and cost.
Upon diagnosis, a uterine septum is treated by noninvasive outpatient hysteroscopic surgical resection which results in significant improvement in subsequent pregnancy outcomes [5, 7, 11]. Ultrasonographic guidance without the aid of hysteroscopy has also been described; 11 patients had postprocedural evaluation of the uterine cavity, 2 patients had a residual septum, and 1 patient had extensive synechiae [12]. Although the number of cases is limited, the results with ultrasonographically guided hysteroscopy appear better than metroplasty without the use of hysteroscopy. Hysteroscopic metroplasty can be successfully performed using microscissors, laser, or electrocautery with comparable improvements in future pregnancy outcomes [7]. The procedure is complete once both ostia are visible simultaneously and when less than 1 cm of myometrium remains [13]. Discontinuing the procedure once a fundal thickness of 8–10 mm is obtained was associated with a normal intrauterine contour on hysterosalpingography [14]. Postoperative hysterosalpingography revealed incomplete resection of the septum when thicknesses of 11–16 mm were used [14].
A prospective, open study including 81 patients undergoing ultrasound-guided operative hysteroscopy for uterine septum or submucosal myoma compared outcomes to that of a historical control group of 45 patients who underwent the same procedure under laparoscopic guidance [15]. All patients in the ultrasound group were successfully treated with a single surgery, and none required conversion to laparoscopy. In contrast, four of the control group patients required additional surgery to resect residual fibroid or septum. Ultrasound guidance allowed the surgeon to accurately determine depth of remaining septum and the outer limit of uterine fundus.
Submucosal Fibroids
Uterine fibroids, the most common benign tumors in females, have a prevalence of 8–18 % in the general population and are often asymptomatic [16, 17]. Symptomatic fibroids can cause pelvic pain, menorrhagia, abdominal fullness, and occasional urinary and bowel symptoms. Fibroid size and location affect the type and degree of patient symptoms and may also have reproductive consequences including pregnancy loss and infertility.
As with uterine septa, resection of submucosal fibroids benefits from ultrasound guidance at time of hysteroscopy. The extent of uterine fibroids’ effect on pregnancy rates and outcomes remains controversial. However, submucosal myomas that significantly distort or encroach on the uterine cavity may lower implantation and pregnancy rates in infertile women undergoing IVF [18]. Several recent studies evaluated the effect of fibroids on in vitro fertilization cycles; the balance of data suggests that pregnancy outcomes and implantation rates are adversely affected by submucosal myomas that enter the uterine cavity but not by subserosal or intramural fibroids that are less than 5–7 cm in size [19–22]. Resection of submucosal fibroids clearly within the uterine cavity is likely warranted in patients with dysfunctional uterine bleeding, infertility, or pregnancy loss who desire to optimize future fertility. A hysteroscopic approach is reasonable if the majority of the fibroid is within the cavity or if subtotal hysteroscopic myomectomy is deemed preferable to abdominal myomectomy. Hysteroscopic resection may be performed using a hysteroscopic morcellator or resectoscope. A resectoscope allows for the use of electrocautery at time of resection but, as a result, requires electrolyte-poor distending media such as mannitol, sorbitol, or glycine. Morcellating devices resect using a rotating blade rather than electrocautery and allow for use of isotonic distending media such as normal saline or lactated ringers which have lower risk for fluid overload and subsequent electrolyte imbalance. The avoidance of electrocautery also has a theoretical benefit of avoiding thermal damage to the myometrium and decreasing chance of future uterine rupture at time of pregnancy. Both methods incur a risk of procedure abortion secondary to bleeding. Prior to starting the hysteroscopy, injection of vasopressin into the cervical stroma can be used to help decrease myoma bleeding. If bleeding is encountered intraoperatively, conversion from morcellator to electrocautery or use of uterine balloon for tamponade and uterine compression can be used to help achieve hemostasis. Lin et al. resected six submucosal myomas under ultrasonographic guidance [23]. All cases were completed in less than 1 h. There were no traumatic complications. None of the patients required laparotomy or blood transfusion. Postoperative electrolyte values revealed no significant change from preoperative values. Menorrhagia and metrorrhagia improved. Postoperative hysteroscopy revealed no intrauterine adhesions, and the endometrium at the operative site appeared normal. Wortman and Dagget used ultrasonographic control to remove large submucosal myomas [24]. These authors claimed that ultrasonography may help prevent perforation and obviate laparoscopy [24].
Synechiae
Asherman’s syndrome, scarring of the endometrial cavity that may have resulted from preexisting infection or uterine instrumentation, may be manifested as hypomenorrhea, amenorrhea, infertility, or recurrent pregnancy loss. Hysteroscopic lysis of adhesions remains the treatment of choice. In severe cases of intrauterine adhesions, it is difficult to determine, without ultrasonography, which part of the cavity is being observed during hysteroscopy. Shalev et al. performed hysteroscopic lysis of intrauterine adhesions using ultrasound (rather than laparoscopic) guidance in 106 women with varying degrees of Asherman’s syndrome. Adhesiolysis was successful in all cases; there were no complications [25]. In cases of moderate-to-severe intrauterine synechiae, ultrasonographic guidance permits the accurate localization of the instruments within the uterus and of myometrial depth. This may result in a greater chance of success in a single surgical procedure with a lower perforation rate. A recent retrospective cohort study including 159 procedures affirms a lower uterine perforation rate for ultrasound-guided (1.9 %) compared to laparoscopic (8.7 %) or blind (5.3 %) hysteroscopic uterine septum resection [17]. Cost was also significantly less for the ultrasound- rather than laparoscopic-guided resections [17].
After significant adhesiolysis, the placement of an intrauterine balloon stent to provide mechanical separation and administration of estrogen to promote endometrial proliferation have been shown to decrease postoperative synechiae formation [19, 26–28]. Initial extent of adhesions has been shown to correlate with adhesion reformation [29]. Both uterine balloons and intrauterine devices have been evaluated for postoperative Asherman’s management. A 1993 study revealed significantly improved outcomes (resumption of menses, increased conception, and decreased need for reoperation) in patients for whom a uterine balloon was placed for 10 days as compared to those who received a nonhormonal copper intrauterine device for 3 months [27]. More recently, investigators are comparing the effectiveness of newer synthetic barrier methods such as Seprafilm and hyaluronic acid gel compounds [30, 31]. Preliminary results are promising; however, larger randomized studies have not yet been performed. Of note, antibiotic prophylaxis is recommended for as long as a foreign intrauterine device remains in place.
The exact regimen and dose of oral estrogen remains unclear. Accepted supplementation ranges from 4 to 6 mg daily (often dosed bi-daily) for 4–10 weeks duration.
Intrauterine Foreign Bodies
The use of ultrasonographic guidance for the retrieval of an intrauterine foreign body has been described [32]. Retained bony fragments after a therapeutic abortion were removed successfully with the use of an ultrasonographically guided resectoscope [3]. Ultrasonography allowed visualization of bone embedded in the myometrium.
Hematometra
As in the case of intrauterine foreign body, ultrasonography can direct the surgeon to the pathologic site within the uterus when this is not evident by direct hysteroscopic vision, as in hematometra. Kohlenberg et al. described five cases of hematometra following endometrial ablation [33]. Ultrasonography was used to guide the hysteroscope to the cornua where these collections were located. Similarly, Goudas and Session described a case of successful treatment of cervical stenosis and hematometra with ultrasonographically guided hysteroscopy [34].
Limitations of the Technique
Replacing laparoscopy with ultrasound at time of hysteroscopic resection of a uterine septum, myoma, or adhesive disease usually decreases potential risk while shortening operative time and maintaining optimal surgical results [25, 35]. However, in a limited number of patients in whom abdominal pathology is suspected, laparoscopy concurrent with hysteroscopy may reveal an intra-abdominal abnormality that can be treated laparoscopically. Additionally, severe retroversion and cul-de-sac adhesions may hinder clear transabdominal ultrasound visualization. Furthermore, diathermy heat may alter tissue or distending fluid by creating microbubbles or produce electrical interference that appears as a “snowstorm” that impedes ultrasonographic imaging [33]. Nonetheless, it is still usually possible to measure myometrial depth, although hysteroscopic localization within the area may be difficult. Additionally, the majority of hysteroscopic procedures can now be performed without cautery using new hysteroscopic morcellating devices.
Summary
For uterine septa, submucosal fibroids, and uterine synechiae, ultrasound guidance helps obtain complete resection while minimizing risk of uterine perforation [24].
Ovarian Cyst and Hydrosalpinx Aspiration
Both ovarian cysts and tubal fluid can be easily aspirated while using transvaginal ultrasound guidance. A needle guide fits on the superior aspect of a standard transvaginal ultrasound allowing the sonographer/surgeon to advance the needle in a predictable, visible plane. Ultrasound color Doppler can be used to confirm an avascular path from the vagina to the ovary or tube.
Ovarian Cyst Aspiration
Large, simple ovarian cysts can form spontaneously, after previous stimulation cycles or as the result of a flare response from GnRH-agonist treatment, and have potential to cause pain or interfere with subsequent stimulation. Usually, cysts rupture or are reabsorbed within 6 months without need for intervention; a recent meta-analysis including eight randomized controlled trials found no benefit to oral contraceptive use to hasten cyst resolution [36]. However, if a cyst is causing significant pain, persists for several months, and grows large enough to pose a significant bleeding or torsion risk, or if time constraints exist for a subsequent cycle, a role remains for cyst aspiration. Cysts may be either functional (hormone-secreting) or nonfunctional. Small (<15–20 mm), nonfunctional (<50 pg/mL estradiol) cysts likely have little effect on fertility treatment; however, functional cysts can deleteriously affect number and quality of retrieved oocytes, fertilization rates, implantation rates, miscarriage rates, and cycle continuation rates [37, 38].
Small, nonfunctional cysts usually do not require intervention. Larger, functional cysts may be managed conservatively either by prolonging downregulation with GnRH agonist prior to stimulation start or by surgical aspiration. In this situation, no clear benefit to surgical aspiration exists [39]. In fact, even for larger (>15 mm), estrogen-producing (>50 pg/mL estradiol) cysts, the majority of evidence suggests no improvement in number of retrieved oocytes, embryo quality, fertilization rate, and implantation and pregnancy rates between women whose cysts were aspirated and those whose cysts were not [39–42]. The deleterious impact of functional cysts on in vitro fertilization cycles persists and, unfortunately, has not been shown to be blunted by cyst aspiration.
Occasionally, however, time pressure, inability to prolong stimulation start, or patient discomfort drive the need for surgical cyst aspiration. Aspirated fluid may be collected and sent to pathology for further cytologic evaluation. A 1992 study including 1,544 oocyte retrievals sent aspirated follicular fluid for cytologic evaluation and found no cases of malignancy [43].
Hydrosalpinx Aspiration
Evidence suggests that the presence of a hydrosalpinx may have a deleterious effect on implantation and pregnancy rates in IVF. This may result from a direct embryo toxic effect of the hydrosalpinx fluid on inhibition of implantation or from a more direct mechanical flushing effect. Implantation rates have been improved by aspiration of hydrosalpinges during the IVF cycle following oocyte aspiration, by salpingectomy or tubal occlusion prior to the IVF cycle, and by extended antibiotic treatment during the IVF cycle [44–46]. Diagnosed hydrosalpinx is usually treated (excised or occluded) prior to initiation of IVF. However, the size of a hydrosalpinges varies during the menstrual cycle [47]. As a result, patients may not have a visible hydrosalpinx prior to stimulation but may accumulate fluid during an IVF cycle. In such situations, mixed evidence exists regarding benefit of aspiration at time of retrieval. A controlled retrospective analysis of 151 women compared implantation rates in patients with hydrosalpinx at time of retrieval and found no benefit to drainage at time of retrieval but confirmed overall reduction in implantation rates in the presence of hydrosalpinx compared to patients without tubal disease [48]. However, a smaller retrospective study (n = 34) reported significantly improved implantation rates (14 % versus 1 % p = .015) with transvaginal aspiration of the hydrosalpinges at time of oocyte retrieval [45]. Possible concerns with this approach include the rapid re-accumulation of hydrosalpinx fluid and risk of pelvic infection caused by the aspiration of the hydrosalpinx. Nonetheless, aspiration of tubal fluid rather than cryopreservation of all embryos may be useful when hydrosalpinges become evident during an IVF cycle as a significant decrease in chance of pregnancy was found only when the hydrosalpinx was evident on ultrasound [49].
Oocyte Retrieval
Although initially performed laparoscopically, oocyte retrieval is now routinely performed using ultrasound guidance due decreased operative risk and a higher rate of oocytes retrieved. Ultrasound-guided aspiration was first described by Lenz in 1981 [50]. Aspiration techniques have included transabdominal, transvesical, and transurethral approaches using sterile-draped abdominal ultrasound and either a guided or freehand needle. The procedure may be performed using mineral oil rather than potentially oocyte-toxic ultrasound gel in a manner similar to amniocentesis [51]. Transvaginal aspiration, first described by Gleicher in 1983, has replaced other routes of aspiration, except when the ovary is not accessible vaginally such as in some cases of Mullerian anomalies [52, 53]. As with all surgical intervention, oocyte retrieval incurs a risk of infection, bleeding, and damage to nearby structures. Such risks are <1 % and are minimized by adequate visualization and proper surgical technique [54]. For transvaginal aspiration, the patient is placed in the dorsal lithotomy position. After sedation is obtained by either a local, regional, or intravenous route, the vagina is prepped with saline lavage or antiseptic to decrease bacterial counts. Prophylactic antibiotics may be given to reduce the occurrence of possible pelvic inflammatory disease particularly in patients with endometriosis [55]; however, peri-retrieval antibiotics have not been shown to affect clinical pregnancy rates [56]. As with cyst and hydrosalpinx aspiration, a transvaginal ultrasound probe is placed in a sterile sheath and fitted with a needle guide through which a single- or double-lumen 16–17 gauge needle is passed to sharply puncture ovarian follicles and aspirate (at 100–200 mmHg) follicular fluid and oocytes. The tip of the needle is etched to increase echogenicity.
Puncture site is determined by visual observation and use of color Doppler for avoidance of vaginal and pelvic blood vessels. The planned course of the needle can be clearly visualized with the biopsy line setting on the ultrasound machine and the probe rotated in such a manner as to avoid blood vessels (see Fig. 21.2). Absence of vasculature in the needle’s course can be confirmed with color Doppler. Additionally, if an ovary is malpositioned, its location can be improved with abdominal pressure or by rotation of the surgical table.
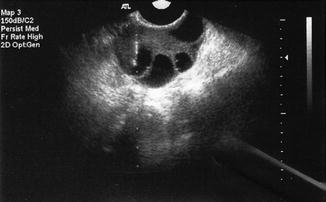

Fig. 21.2
Transvaginal oocyte retrieval (Dotted line denotes “needle biopsy line”)
The decision to use a single- or double-lumen needle depends on the physicians desire to flush aspirated follicles with culture media to attempt to increase oocyte yield. Flushing requires a double-lumen needle while direct aspiration can be performed using a single-lumen needle. Proponents of flushing contend that it increases oocyte yield and may be clinically significant in poor responders for whom even one additional oocyte may improve pregnancy potential. Waterstone and Parsons found a 20 % increase in oocyte yield in the first three flushes after direct aspiration in 50 patients for whom oocyte yield was quantified after direct aspiration, after 3 flushes, and after 6 flushes [57, 58]. However, a Cochrane review including four randomized trials with number of study participants ranging from 4 to 100 found no significant difference in oocyte yield or clinical pregnancy rates for flushed compared to directly aspirated follicles[58]. Flushing required statistically significant increased operative time and analgesia use [58].
After retrieval completion, assessment of hemostasis is essential. A survey of the pelvis with Doppler ultrasound before and after the retrieval should be performed to look for fluid pockets and for active sources of bleeding. If observed, focused bimanual pressure usually suffices to tamponade active bleeding. After abdominal hemostasis has been confirmed, a speculum should be placed in the vagina to inspect puncture sites. Bleeding vaginal sites also usually stop either with direct pressure, vaginal packing, application of an atraumatic clamp for focal direct pressure, or rarely with placement of a nonreactive vaginal suture.
Ovarian hyperstimulation is a complication of assisted reproductive technology (ART). Ascites results from increased vascular permeability. Paracentesis may decrease pain, shortness of breath, and oliguria [59]. Both transvaginal and transabdominal aspiration have been described under ultrasound guidance (see Fig. 21.3) [59].
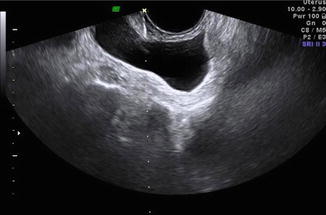

Fig. 21.3
Transvaginal aspiration of ascitic fluid in patient with ovarian hyperstimulation syndrome. Needle visualized along dotted biopsy line
Embryo Transfer
Ultrasound guidance has potential to play an integral part in IVF embryo transfer. Pregnancy rates may be affected by embryo transfer techniques in IVF. Embryo transfer techniques that have been demonstrated to have an effect on pregnancy outcome include type of catheter [60], trial transfer attempt prior to the IVF cycle [61], technique of catheter loading [62], time to withdraw catheter [63, 64], and atraumatic transfer [65]. Abdominal ultrasound guidance for embryo transfer has been evaluated by several investigators.
Embryos are loaded into a catheter with the placement marked by air bubbles. Under abdominal ultrasound guidance with a full bladder, the placement of the catheter can be confirmed and the air bubble visualized when injected into the uterus (see Fig. 21.4




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree