8 Ultrasound of the retroperitoneum and gastrointestinal tract
Normal anatomy
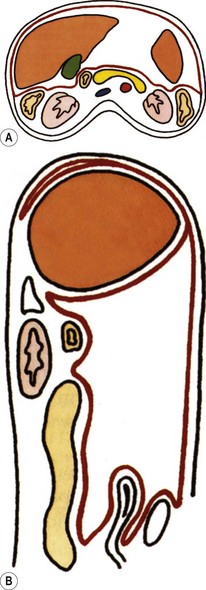
The retroperitoneal space contains the kidneys and ureters, adrenal glands, pancreas and duodenal loop, great vessels and the ascending and descending portions of the large bowel, including the caecum (Fig. 8.1).
The abdominal aorta
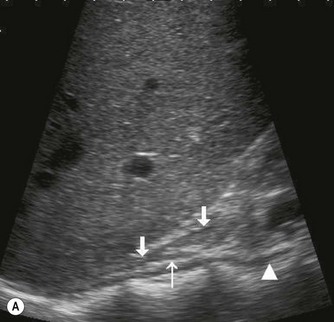
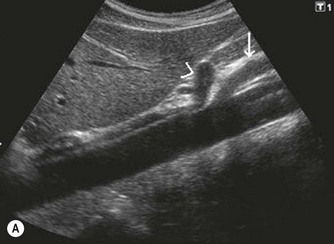
The proximal abdominal aorta can usually be visualized in the midline, inferior to the xiphisternum, by using the left lobe of liver as an acoustic window. The coeliac axis and SMA are easily demonstrated in LS, arising from its anterior aspect (Fig. 8.2); the coeliac axis branches – the main hepatic and splenic arteries – are better appreciated in a transverse section. Just below this level, the origin of the SMA is seen with the renal arteries inferior to this.
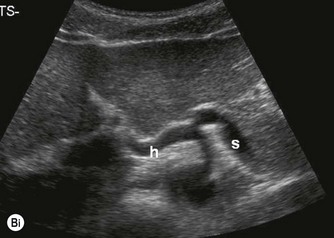
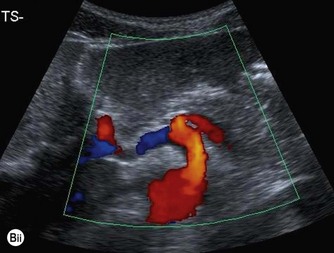
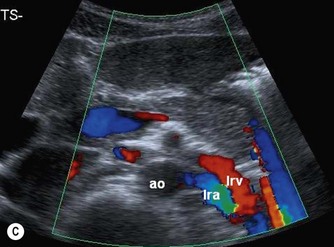
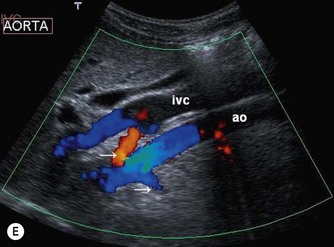
(Bii) Colour Doppler demonstrates the direction of flow with respect to the transducer.
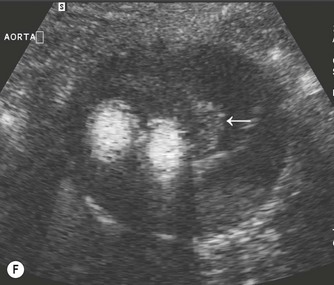
(D) A coronal plane, from the patient’s right side, demonstrates the aortic bifurcation.
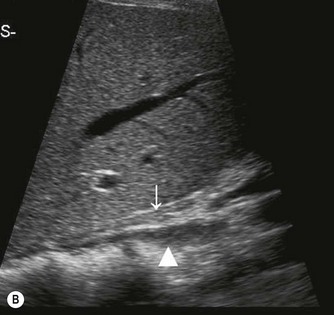
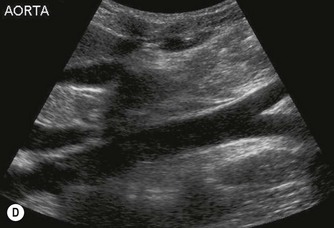
The distal abdominal aorta runs more anteriorly, towards the bifurcation. Here, bowel gas may obscure the structures in sagittal section. A coronal approach overcomes this problem: from the patient’s left side the aorta can be demonstrated using the left kidney as an acoustic window, and from the patient’s right side, the right lobe of liver affords good access (Fig. 8.2D, E). A coronal view is also useful in displaying the origin of the renal arteries.
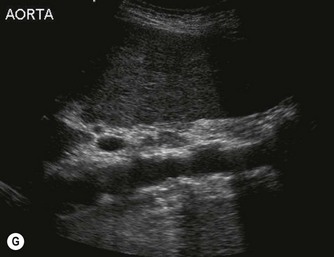
The aorta often becomes ectatic and tortuous with age, and it is not unusual to detect considerable calcification of the walls (Fig. 8.2G).
Aortic aneurysm
Abdominal aortic aneurysm (AAA) is a common and potentially deadly condition. The risk factors for AAA include male gender, age over 65, smoking and a family history. AAA is found in up to 10% of men aged 65 and over. The risk of aneurysm rupture increases with diameter, increasing dramatically when it reaches 6 cm, with a 1-year mortality of 50%.1
Screening of the high-risk population with ultrasound is increasingly being adopted. Small aneurysms may then be monitored to allow timely treatment (open or endovascular repair) with a subsequent fall in mortality.2,3 It is generally accepted that repair is indicated in any aneurysm of over 5.5 cm diameter due to the imminent risk of rupture.4
Ultrasound appearances and measurements
Most aneurysms are associated with atherosclerosis, which weakens the media of the wall, causing the vessel to dilate and eventually rupture. The aneurysm may be fusiform or saccular (Fig. 8.3). Blood flow within it is turbulent, and the slow flowing blood at the edges of the vessel tends to thrombose.
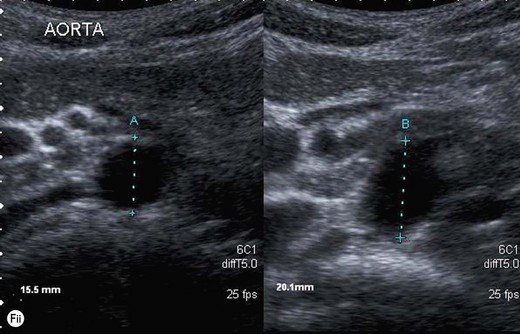
The true maximum diameter of the aneurysm should be ascertained in LS, where the calipers can be placed perpendicular to the walls for an accurate diameter at the widest part of the aneurysm. This is a reliable and reproducible measurement in trained hands. CT measurements and ultrasound measurements done in a transverse plane tend to overestimate the diameter, as the aorta often runs obliquely (Fig. 8.2F). When the aorta is scanned in TS, care must be taken to keep the transducer perpendicular to the (often tortuous) axis of the vessel to avoid inaccurate measurements. The ability of ultrasound to locate the correct plane for measurement, regardless of vessel tortuosity, is an advantage over CT, which may overestimate the size of the aneurysm in an axial plane.
Complications of aortic aneurysm
Leakage of an aneurysm may cause retroperitoneal haematoma, but CT is usually more reliable in detecting leaks than ultrasound. CEUS has had success in identifying leaks following AAA repair, and has been reported to be more sensitive than CT in this group (Fig. 8.3F).5,6 This has the added advantage of reducing radiation dose due to CT, especially in patients who are regularly monitored with imaging.
The inferior vena cava
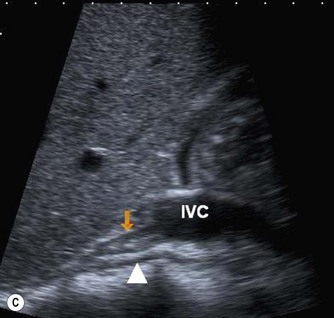
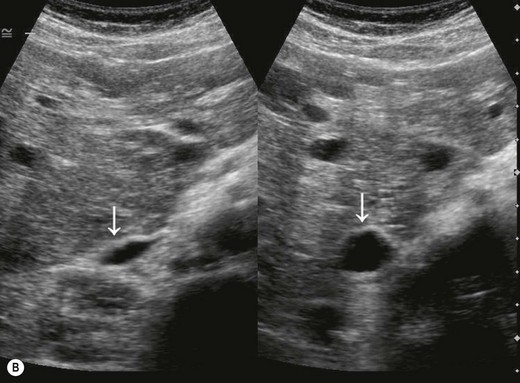
The normal IVC has thinner walls and a more flattened profile than the aorta, and its lumen alters with changing abdominal pressure, for example during respiration the lumen decreases on inspiration, or with the Valsalva manoeuvre (Fig. 8.4). The main renal veins may be seen in TS, entering the IVC just below the level of the pancreas.
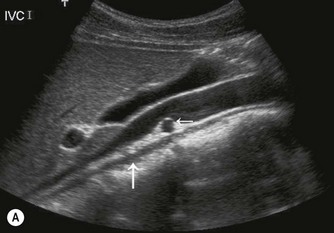
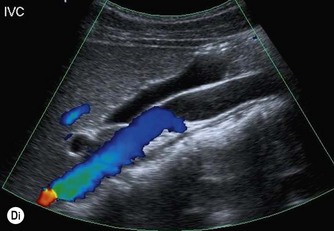
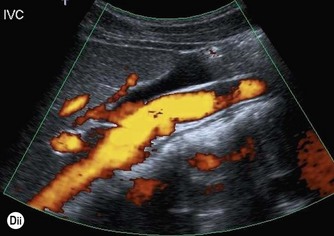
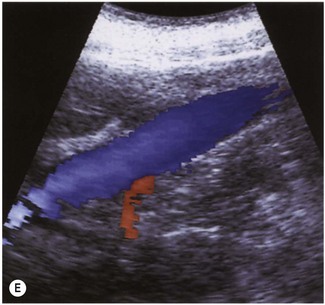
(C) IVC at the level of the confluence of the hepatic veins, just beneath the diaphragm.
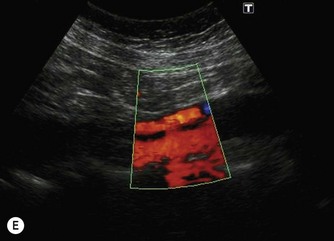
(E) The RRV (in red) is seen draining into the IVC on colour Doppler.
Pathology of the IVC
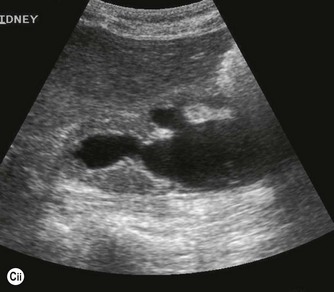
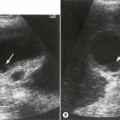
Thrombus in the IVC may be due to benign causes, or the result of tumour. It is not usually possible to tell the difference on grey-scale appearances alone, but vascularity may be demonstrated on power or colour Doppler within tumour thrombus, and the clinical history is helpful. Tumour thrombus invades the renal vein and enters the IVC in around 10% of renal carcinoma cases. Tumour thrombus from hepatic or adrenal masses can also invade the IVC (Fig. 8.5).
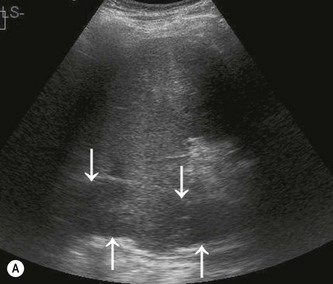
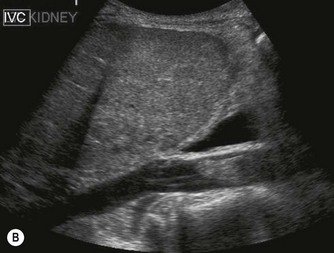
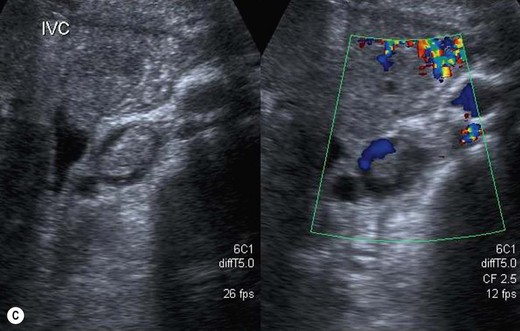
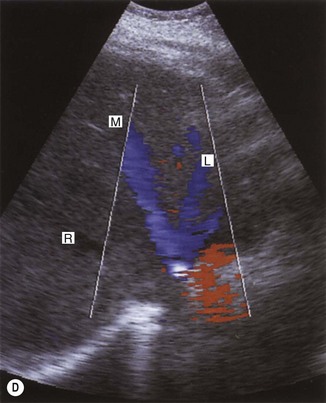
Fig. 8.5 • (A) Tumour thrombus, from a left renal carcinoma completely occludes the IVC (arrows).
(B) Advanced renal carcinoma. The IVC contains tumour thrombus.
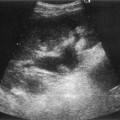
Coagulation disorders, which cause Budd–Chiari syndrome (see Chapter 4) predominantly affect the hepatic veins, but may also involve the IVC (Fig. 8.6). Patients may require the insertion of a caval filter, which is performed under X-ray guidance, but may be monitored for patency using ultrasound with Doppler. Dilatation of the IVC is a finding commonly associated with congestive heart failure, and is frequently accompanied by hepatic vein dilatation.
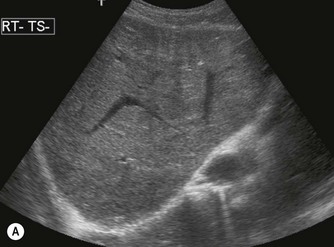
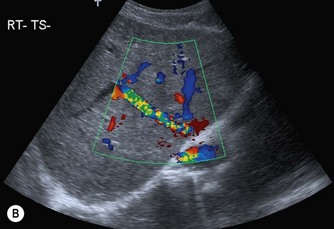
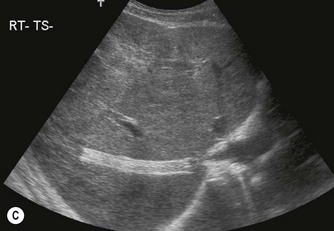
Fig. 8.6 • (A) Budd–Chiari syndrome, with an occluded RHV.
(B) Confirmed with colour Doppler.
(C) A stent has been inserted under angiographic guidance to restore flow in the vein.
Compression of the IVC by large masses is not uncommon. This may be due to retroperitoneal masses, such as lymphadenopathy, or liver masses such as tumour or caudate lobe hypertrophy. Colour or power Doppler is particularly useful in confirming patency of the vessel and differentiating extrinsic compression from invasion. Insertion of metallic stents may be performed under angiographic control to maintain the vessel patency, particularly if the compression is due to inoperable hepatic metastasis (Fig. 8.6).
Tumours of the IVC are rare. Leiomyosarcoma is a primary IVC tumour, appearing as a hyperechoic mass in the lumen of the vein.7,8 This may cause partial or complete obstruction of the IVC resulting in Budd–Chiari syndrome. In partial occlusion, the hepatic veins and proximal IVC may be considerably dilated. Resection of the tumour, with repair of the IVC, is possible provided the adjacent liver is not invaded.7
The adrenal glands
Normal appearances
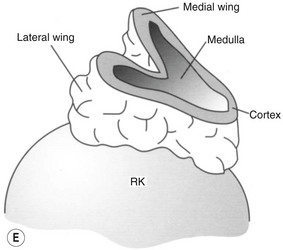
The normal adrenal glands can be seen on ultrasound in the vast majority of patients,9,10 if you know where and how to look. Each adrenal gland is constructed with a central fold or ridge, which points anteromedially, from which extend two thin ‘wings’ of tissue – a medial and a lateral wing (Fig. 8.7).