The classic multiple sclerosis variants including Devic’s neuromyelitis optica (NMO), Balo’s concentric Sclerosis, Schilder’s disease, and Marburg MS are both interesting and instructive from a disease pathophysiology perspective. Although rare, the variants are important as they often arise in the differential diagnosis for severe, acute demyelinating disease, including MS and acute disseminated encephalomyelitis. In the case of NMO, an originally unsuspected and entirely new pathophysiology based on water channels has been described, only after the recent original description of the more specific diagnostic test for NMO based on serum immunoglobulin.
The so-called variants of multiple sclerosis (MS) although relatively rare are clinically important as they cause considerable diagnostic uncertainty and, sometimes, misdiagnosis. As new more aggressive and more targeted treatments for MS and its variants are developed, accurate diagnosis becomes more critical. The variants of MS are also instructive as they force imagers, clinicians, and basic scientists to consider the factors that result in variant imaging for these unusual clinical/immunologic presentations. One variant, Devic’s Neuromyelitis optica (NMO), has been central to discovery of an entirely new pathophysiology involving water channels, which was revealed in recent years.
Controversies and history that are central features in many discussions of the MS variants have been previously addressed. For this article, the authors’ approach is to discuss the currently clinically most important MS variants, ie, those that are most likely to be seen or considered in clinical practice, including NMO, Balo’s concentric sclerosis (BCS), Marburg MS, and Schilder’s disease. Acute disseminated encephalomyelitis (ADEM) is briefly discussed as its clinical presentation, imaging, and pathologic features frequently elicit a discussion of the MS variants Table 1 .
| Relapsing MS | Acute Disseminated Encephalomyelitis | Devic’s Neuromyelitis Optica | Balo’s Concentric Sclerosis | Schilder’s Disease | Marburg MS | |
|---|---|---|---|---|---|---|
| Age | Childhood to adult | Childhood to adult | Childhood to adult | Childhood to adult | Predominantly children | Typically in young adult |
| Typical clinical course |
|
|
|
|
|
|
| MR imagingbrain |
|
|
| Lamellar lesions in isolation or accompanying typical MS-like lesions |
|
|
| MR imagingspinal cord |
|
|
| MS-like | ||
| Pathology |
| Perivenous inflammation, demyelination |
| Concentric zones of normal myelin alternating with demyelination | Severe myelin loss; large, well demarcated, bilateral white matter plaques involving cerebral hemispheric white matter | Severe acute myelin loss with numerous Luxol fast blue-positive macrophages; widely distributed, becoming confluent. |
Devic’s neuromyelitis optica
NMO has been classified as an inflammatory, demyelinating disease with features that overlap with MS. However, with recent discoveries that related initially to serum markers (immunoglobulins) and subsequently to the specific targets of these immunoglobulins (aquaporins), NMO is now accepted as distinct from MS. The NMO serum biomarker, an autoantibody called NMO-IgG, first aroused interest as it showed promise as a more specific measure for NMO, potentially distinguishing NMO from MS, but it also distinguished MS from ADEM and possibly Asian forms of MS with stronger spinal-optic presentations. NMO-IgG was subsequently found to specifically target aquaporin-4 (AQP4), the most abundant water channel in the central nervous system (CNS). The current hypothesis is that action at the AQP4-rich sites in the CNS may be an underlying pathophysiologic mechanism for NMO, in contrast to the mostly unknown pathophysiologic mechanisms in MS and the other MS variants.
Clinical
NMO is characterized clinically by acute visual loss, often bilateral, and/or acute transverse myelitis, but with the visual and spinal cord signs and symptoms often presenting nearly simultaneously. The visual symptoms of NMO usually precede the spinal symptoms, but the reverse is not uncommon. Severe visual symptoms with blindness may become total and permanent within a few days and bilateral optic neuritis is most common. This is in contrast to MS where bilateral optic neuritis is relatively uncommon and the initial visual compromise is usually limited and reversible. In patients who present with visual system difficulties, transverse myelitis characteristically develops within a few weeks, with severe paraplegia, sensory loss with a distinct level, and sphincter disturbances. Fixed weakness from onset, rather than improvement over time after the initial event is the typical course, again in contrast to MS where weakness is frequently reversible in the early stages and where the spinal cord involvement, both clinically and by imaging, is usually partial transverse (asymmetric and/or limited) myelitis. The interval separating the visual and spinal syndromes may be days or weeks, but in most cases is within 3 months. The clinical course following the presentation of NMO is variable but is most often monophasic, that is, without recurrent attacks (but with fixed deficits), or, less frequently, multiphasic with the patient experiencing multiple severe relapses with step-wise neurologic deterioration. NMO may also follow an acute progressive and potentially fatal course with respiratory failure and death after cervical myelitis. Rarely, there can be complete clinical recovery without relapse. Prognosis is poor compared with classic MS, with most patients left with severe visual loss or inability to ambulate without assistance within five years of onset.
Neuropathology
NMO is characterized pathologically by considerably more tissue destruction and greater loss of axons than is seen in typical MS, with necrotizing demyelination in the spinal cord and optic nerves. The tissue involvement often extends over numerous spinal cord segments and the cord may be swollen. Over time there may be considerable shrinkage of the cord and cavitation due to tissue destruction. NMO may show a greater B cell component, more prominent eosinophilic and neutrophilic infiltrates, complement activation, and vascular fibrosis, all rare in typical MS, along with more MS-like features including T cell infiltrates and the presence of macrophages.
Imaging
Spinal cord
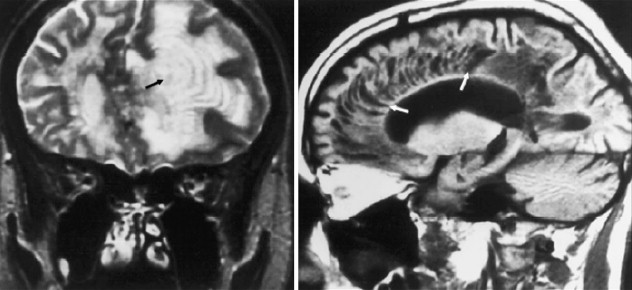
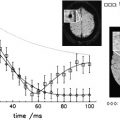
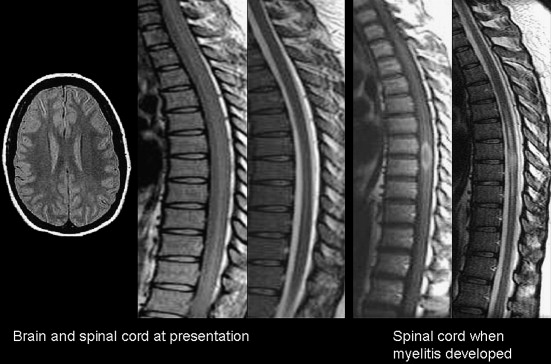
By magnetic resonance (MR) imaging, NMO lesions of the spinal cord tend to be vertically extensive, often exceeding three vertebral segments ( Fig. 1 ), and a lesion on axial imaging will often extend across much or all of spinal cord area. Both the gray and white matter as well as the central cord may be abnormal, and the spinal cord may be swollen. This characteristic appearance is in contrast to typical MS where spinal cord involvement, although also vertically oriented, is usually two segments or fewer in length, and on axial images, lesion is asymmetrically distributed across the spinal cord cross section. Although the spinal cord can be swollen in MS in the acute stages of a focal lesion, relative to NMO, the swelling is most often mild, if perceived at all by visual criteria.
Another difference between MS and NMO is the limited hypointensity of spinal cord lesion on T1-weighted imaging even in acute MS lesions, compared with the often striking T1-hypointense changes that are seen in NMO. Chronic T1-hypointensity in the spinal cord (T1-black holes) are also notably absent in MS, but they occur in NMO, probably related to more severe and more extensive injury, including necrosis by histopathology. In NMO, there may be longitudinally extensive and central enhancement of the spinal cord as compared with MS where enhancement may be difficult to detect, is more spotty and ill defined, and more fleeting.
Spinal cord atrophy does occur frequently in MS, but most often by visual criteria, it is apparent as localized and segmental. In NMO, over time, spinal cord atrophy may be readily apparent, and may occur over long segments.
Anterior visual system
Imaging findings in the optic nerves and optic chiasm, including T2-hyperintensity, swelling or enhancement, may be less helpful in distinguishing NMO from typical MS. When present, lesions follow similar patterns to the spinal cord in extent and intensity, beyond what is often observed in MS.
Brain
Despite the striking extent and distribution of clinically or imaging–apparent lesion in the anterior visual pathways and spinal cord, patients with “pure” NMO show remarkably limited neurologic signs or symptoms or demyelination in other regions of the CNS. Most series suggest that the majority of cases will be characterized by a normal or near normal brain MRI at presentation (see Fig. 1 ), and, when positive, with only limited nonspecific white matter abnormalities. However, other series suggest that these nonspecific patterns are not so rare. The nonspecific T2-hyperintensities that are described in NMO may not abut the ventricular surfaces, and they are not accompanied by acute or chronic T1-hypointense lesions that are more typical in MS. On follow-up, cases of NMO are less likely to accumulate new T2-hyperintensites, in contrast to typical MS where new lesions are frequent.
Support for a different lesion distribution in MS and NMO comes from studies of the normal appearing white matter (NAWM), where magnetization transfer ratios tend to be normal for NMO, in contrast to MS where the NAWM is more typically abnormal.
Another NMO lesion by imaging?
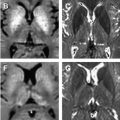
Cases of NMO have been observed that meet NMO diagnostic criteria clinically, yet have relatively specific patterns of brain involvement beyond the optic nerve. In these cases, T2-hyperintense lesions were observed in the hypothalamus, bilateral periventricular surfaces surrounding the third ventricle, in the region of area postrema, and more symmetrically than expected for MS around the base of the fourth ventricle ( Fig. 2 ). This distribution was noted to overlap with regions of high AQP4 expression in the periependymal regions, potentially linking disturbances of water homeostasis and the imaging findings. The lesions in brainstem have been noted to be relatively assymptomatic and reversible.
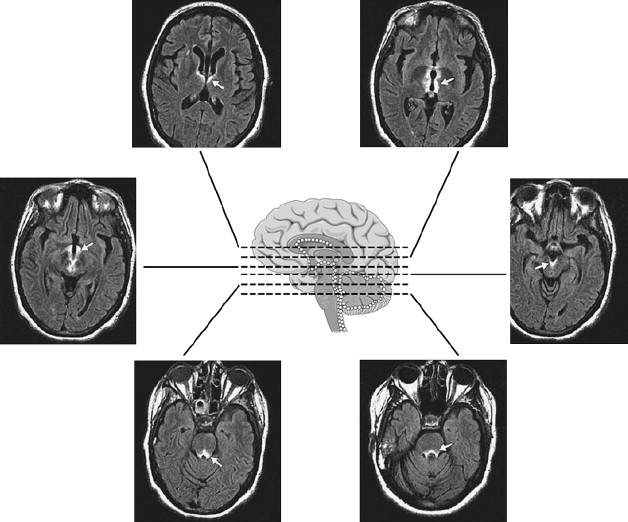
A current hypothesis encompasses two basic pathologies in NMO, both of which are associated with loss of AQP4 immunoreactivity, the most prevalent pathology involving the spinal cord and optic nerves, with AQP4 loss in the context of vasculocentric immune complex deposition, active demyelination and vascular hyperplasia with hyalinization. The less frequent pathology in the spinal cord and medulla extending into the area postrema appears to be highly inflammatory. In the latter, AQP4 loss was associated with vasculocentric IgG and IgM deposits, complement activation, and tissue rarefaction, but there was no evidence of demyelination.
Current Diagnosis
Despite remarkable improvements in laboratory criteria and stronger imaging criteria, no single criterion is specific for NMO, including the current serum autoantibody test, which is based on NMO-IgG seropositive status. Cerebrospinal fluid (CSF) oligoclonal bands, less likely in NMO than MS, are not specific or sensitive. NMO-IgG is moderately sensitive, and increasingly accepted as moderately or strongly specific, but it does not provide a definitive diagnostic test in itself. Consequently, diagnostic criteria based on multiple factors have been proposed, requiring optic neuritis and acute myelitis (clinical considerations), and two of three supportive criteria which can include MR imaging contiguous spinal cord involvement extending over three vertical segments, a brain MR imaging study not meeting MS diagnostic criteria, or NMO-IgG seropositive status.
Balo’s concentric sclerosis
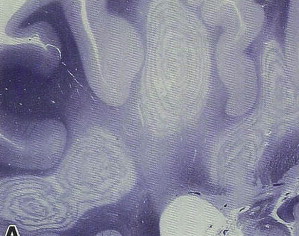
BCS shows a peculiar pattern of pathology (most often in the cerebral hemispheric white matter) consisting of a concentric configuration of alternating bands of white matter of different pathology, with relatively preserved myelination alternating with regions of demyelination ( Fig. 3 ). The BCS pathologic patterns can be found throughout the neuroaxis including the spinal cord, optic chiasm, brainstem, cerebellum, and supratentorial brain.
Clinical
The original description and early literature concerning BCS emphasized an aggressive form of demyelination with poor outcome. Disease typically was described as progressing over weeks to months, with severe disability or death as the typical outcome; clinical symptoms include headache, aphasia, cognitive or behavioral dysfunction, and/or seizures.
Because these cases were diagnosed at autopsy, there was a strong case selection bias toward worst-case outcomes. Using MR imaging, BCS lesions have been found that co-exist with typical MS-like lesions and in patients with a typical MS course, although some patients are severely affected. Reports of BCS–like cases by imaging often have favorable clinical and imaging outcomes.
BCS lesions have been found rarely to develop after a typical relapsing-remitting course of MS, and Balo-like bands have been described along the periphery of acute MS plaques. Co-existence of BCS lesions with typical MS-like (nonconcentric) lesions is now well recognized in the neuropathology literature.
The clinical course of the more “benign” BCS has been described as monophasic with resolution of pathology and clinical findings over time, and as MS-like with a multiphasic but self-limited course, and responsive to therapy. A detailed review of the literature is presented in Mowry and colleagues.
Neuropathology
Although the early pathology literature suggested that the abnormal bands (intermingled with normal bands) were the result of partial remyelination, more recent evidence suggests that the abnormal bands are more often areas of incomplete demyelination alternating with preserved myelin. The mechanisms responsible for this peculiar pathology remains a mystery, but the visually fascinating BCS patterns, although rare, have attracted much attention. It is thought that the process that gives rise to the concentric layers may arise through inflammatory demyelination. In some individuals, a zone of protective preconditioning of oligodendrocytes is located in the surrounding layer of immediately adjacent white matter. This zone is therefore preserved from demyelination as the inflammatory process spreads outward. However, inflammation reaches the next, more peripheral region, which contains susceptible oligodendrocytes, and this zone is susceptible to injury.
Imaging
BCS lesions are readily identified on proton density or T2-weighted images, but the concentric pattern may also be apparent on T1-weighted images. The rings in BCS may show contrast enhancement; the enhancing regions are thought to correspond to zones of demyelination. Synchronously enhancing, sequentially enhancing, and transiently enhancing rings have been reported.
BCS may present as classic large tumors in isolation associated with a fulminant clinical course, or as cases in which less impressive BCS–like lesions co-exist with typical MS lesions ( Fig. 4 ). There is also increasing recognition of borderline BCS–like lesions with only a few lamellae or rings, but the relationship of these to more classic Balo’s patterns is uncertain ( Fig. 5 ).