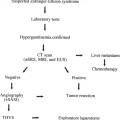
Vascular Access for Hemodialysis: A Nephrologist’s Perspective
The number of patients with chronic renal failure progressing to end-stage renal disease (ESRD) is increasing in the United States by 11 % per year.1 This patient population has increased from nearly 8000 patients in 1972 to more than 250,000 patients in 1998. This growth has been fueled by the aging of the American population combined with technical improvements in other areas, such as cardiovascular care, allowing patients to live longer and thus develop kidney disease.
The two leading causes of chronic renal failure are diabetes and hypertension, accounting for 39% and 28.4% of the U.S. population on dialysis.1 Other less common etiologies of ESRD include glomerulonephritides; genetic diseases, such as Alport’s syndrome and autosomal dominant polycystic kidney disease (ADPKD); and congenital anomalies, such as vesicoureteral reflux and posterior ureteral valves.
The mean age of patients on dialysis is 59.9 years.1 It is anticipated that the mean age will become higher as the number of patients on dialysis who are older than 65 continues to climb.
Patients with ESRD can be treated with hemodialysis, peritoneal dialysis, or renal transplantation. Of the three, renal transplantation is the most physiologic and offers the patient the best chance for long-term survival. Unfortunately, donor shortages and underlying comorbid conditions make either form of dialysis the only initial choice for most patients. In the United States, 83% of patients elect hemodialysis over peritoneal dialysis.
Unfortunately, despite tremendous technical advances in the dialysis field, the mortality and morbidity rates for a patient with ESRD on dialysis are significant. Data from the United States Renal Data Systems (USRDS) indicate that for the years 1993 through 1995, the mortality rate for patients with ESRD was 24 to 25%.l Patients on dialysis averaged one or two hospital admissions annually, accounting for an average of 17 to 22 patient days per year. This high hospitalization rate carries an annual cost of more than $60,000 per patient per year.
Because of these dismal statistics, a concerted effort is being made by the renal community to improve the care of patients on maintenance dialysis. An example of this effort has been the recent publication of the Dialysis Outcomes Quality Initiative (DOQI).2 This effort, spearheaded by the National Kidney Foundation (NKF), involves health care professionals from the specialties of nephrology, radiology, and surgery. The goal of this project was to develop evidence-based guidelines for the care of dialysis patients. These guidelines, which focus on dialysis adequacy, dialysis access, and the management of anemia, have been endorsed by the relevant professional societies and have become the standard of care for patients receiving dialysis in the United States. This chapter focuses on one aspect of dialysis care: vascular access.
 Pre-ESRD Years
Pre-ESRD Years
Historically, “dialysis care” began the moment the patient required his or her first dialysis treatment. Over the last several years, there has been a strong emphasis on pre-ESRD care. Numerous studies have documented a decrease in morbidity and significant cost savings associated with earlier referral of the patient with chronic renal failure to a nephrologist.3,4 Issues that should be addressed during this period include management of anemia, the treatment and prevention of hyperparathyroidism, nutritional education, modality selection, and—relevant to this chapter—dialysis access planning.
Patients who choose hemodialysis should be evaluated for vascular access when the serum creatinine is greater than 4 mg/dL or when dialysis is thought to be needed within the next 12 months. As discussed elsewhere in this book, three types of vascular accesses are available for patients on hemodialysis: an arteriovenous native fistula (AVNF), synthetic bridge grafts (SBG), and dual-lumen catheters. Without question, AVNF is the preferred access because of its greater longevity with fewer complications.
Advanced planing allows the team caring for the patient adequate time for the creation and maturation of an AVNF. Unfortunately, most patients on dialysis require more than one dialysis access in their lifetime. Therefore, access planning includes more than the initial access; rather, attention should focus on a lifetime plan for the patient.
The initial access should be placed in the nondominant arm in the most distal location. The nondominant extremity is chosen to avoid significant functional loss of the hand in the unlikely event that creation of the access is complicated by vascular steal or mechanical nerve damage. The distal location is chosen so as not to compromise alternative sites for future access procedures.
Every attempt should be made to place a radial–cephalic fistula. If the physical examination or venography indicates questionable arterial inflows or venous outflow obstruction, a brachial–cephalic or a brachial–basilic vein AVNF should be considered.
Unfortunately, because of advanced age and comorbid conditions, or as a result of previous phlebotomy from veins in a nondominant arm, many patients are not candidates for an AVNF. In such cases, a synthetic bridge graft should be created in the nondominant arm in the most distal location.
Unlike an AVNF, synthetic bridge grafts are placed only 6 to 8 weeks before the first dialysis because these shunts have a limited functional life independent of whether they are used for dialysis. Once a patient is identified as needing a SBG, the patient should be carefully instructed not to allow phlebotomy or blood pressure measurements proximal to the proposed access site to avoid damage to the venous circulation.
 Dialysis Years
Dialysis Years
During the time the patient is on dialysis, the focus for the health care team is on providing adequate dialysis while correcting the physiologic abnormalities that accompany chronic renal failure. The vital lifeline for a hemodialysis patient is his or her vascular access. Most patients in the United States currently do not have an AVNF; therefore, these patients must contend with the recurrent issue of vascular access thrombosis. Historically, these access devices were allowed to thrombose before any intervention was attempted. It was widely believed that as long as blood was flowing through the access, it must be working. This thinking ignored the pathophysiologic consequences of neoitimal hyperplasia, namely intragraft recirculation and decreased graft blood flow. During the 1990s increasing emphasis was placed on early detection of impending graft thrombosis. The rationale for early intervention was to optimize dialysis adequacy and decrease the cost associated with vascular access thrombosis and revision. Table 23–1 lists the various diagnostic tests commonly used to diagnose preemptively impending graft failure.
The measurement of urea recirculation is one of the oldest diagnostic tests used by nephrologists to determine the patency of a vascular access. Recirculation occurs within the dialysis access when blood flow that is returned through the venous needle encounters a proximal vein obstruction. This obstruction may be the result of either a thrombus formation or the presence of a stenosis within the graft, at the vein graft anastomosis, or in a more proximal vein. As the flow of blood encounters an obstruction, the pressure within the vein increases, resulting in retrograde blood flow into the arterial end of the graft, thus creating recirculation of blood between the arterial and venous needle (Fig. 23–1). Recirculation of urea can be calculated using the following formula:
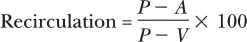
where P is the concentration of urea from a peripheral vein on the contralateral arm,
A is the concentration of urea obtained via the arterial needle,
and V is the concentration of urea obtained from the venous needle.
Recirculation of urea should not exceed 15% in a native fistula or synthetic bridge graft. If elevated recirculation values are documented, a fistulogram should be performed to determine the site of anatomic obstruction. This study should include views of the ipsilateral arm and central circulation.
Urea recirculation Dynamic venus pressure monitoring Static pressure monitoring Poor solute clearance Doppler ultrasound Physical examination Dilution ultrasound |
FIGURE 23–1. Diagram of PTFE bridge graft with venus stenosis and associated recirculation.
Venous-pressure monitoring measures pressure within the venous drip chamber on the dialysis machine. This pressure is determined by blood flow through the dialysis circuit, resistance to flow within the dialysis circuit, and resistance within the venous access limb. Blood flow and resistance within the dialysis circuit remains relatively stable; therefore, any change in venous pressure theoretically results from increased resistance within the native vein, suggesting obstruction. Using such a theory, Schwab et al5 arbitrarily chose a dynamic venous pressure greater than 200 mg Hg at a blood flow of 200 mL per minute to study prospectively the question of whether an elevated venous pressure correlates with a proximal stenosis. In this study, 58 patients were identified as having an elevated venous pressure, and 50 of these patients had a significant stenosis noted by fistulography. Likewise, using a venous pressure cutoff of 150 mm Hg, Levy and colleagues6 identified significant stenoses in 13 of 29 patients studied.
Expanding on these methods, Besarab and colleagues7 used static venous pressure measurements in an attempt to calculate intragraft pressures more accurately. These authors documented that this technique eliminated possible confounding variables, such as needle size, hematocrit, and blood pump speed. When measuring static venous pressure, the blood pump speed is reduced to 0. The tubing between the dialyzer and venous drip chamber is clamped. The venous pressure is documented 30 seconds after turning the blood pump to 0. Simultaneous measurement of the mean arterial pressure (MAP) is made using a conventional sphygmomanometer. Correcting for the height difference between patient’s access and the venous pressure transducer, the intraaccess pressure can be calculated by using the following formula:
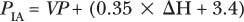
Then the ratio of PIA to MAP is determined. In a prospective study of 33 patients in six different centers, a PIA:MAP ratio greater than 0.5 correlated with a graft blood flow of 1222 ± 112 mL per minute, whereas a PIA:MAP below 0.5 correlated with a blood flow of 555 ± 45 mL per minute. These authors demonstrated that this test was efficient, reproducible, and had fewer confounding variables compared with dynamic pressure monitoring, as suggested by Schwab and colleagues.5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree