CHAPTER 31 Vascular and Hematopoietic Neoplasms
The focus of this chapter is on tumors that are vascular or of blood cell origin. These include hemangioblastoma, hemangiopericytoma, primary central nervous system (CNS) lymphoma, intravascular lymphoma, leukemia, and plasmacytoma. These neoplasms combined make up fewer than 10% of CNS tumors.
HEMANGIOBLASTOMA
Hemangioblastoma is a benign vascular tumor composed of endothelial and stromal cell components that can occur throughout the neural axis. The majority of these tumors are sporadic (75%), with the remaining associated with von Hippel-Lindau (VHL) disease.1 It is also referred to as capillary hemangioblastoma and is the most common primary intraparenchymal tumor to occur in the posterior fossa in adults.
Epidemiology
Sporadic hemangioblastomas have their onset at an average age of 35 years and are more common in men. In patients with VHL they present an average of 10 years earlier. Sporadic cases are usually solitary lesions that can recur, whereas lesions associated with VHL are often multiple. There is no difference between sporadic and VHL-associated tumors with respect to recurrence or dissemination.1
Clinical Presentation
The clinical presentation often includes headache, ataxia, nausea, vomiting, and focal neurologic deficits. Symptoms depend on the size and location of the tumor. Hemangioblastomas can be found throughout the neural axis but are most common in the posterior fossa (44%-72% in the cerebellum, 13%-44% in the spine).2–4 Patients may also present with subarachnoid hemorrhage.
The majority of mass effect is from the associated cyst rather than the tumor; the larger the tumor, the more likely it is to produce an associated cyst. Cysts grow at a rate several times that of the solid tumor and reach a volume almost always several times the volume of the causative tumor.5
In VHL, ocular hemorrhage secondary to a retinal lesion is often the first manifestation of disease.
Secondary polycythemia may be seen secondary to increased erythropoietin production.
Pathophysiology
Tumor growth is variable, and both the solid and the cystic component can cycle through periods of growth and stability.5
The VHL gene is a tumor suppressor gene on chromosome 3 (3p25-26) found in patients with VHL disease.6
Local growth factors such as vascular endothelial growth factor, placental growth factor, epidermal growth factor, and platelet-derived growth factor, as well as their respective receptors, have been shown to be elevated in patients with hemangioblastomas.7–9
Imaging
CT
The most common finding (60%) is a cerebellar cyst with a mural nodule isodense to brain on nonenhanced CT. The mural nodule is often found adjacent to the pial surface. The lesion can be purely solid. On postcontrast imaging, the solid component enhances avidly. Faint marginal enhancement may be seen around the cyst that likely reflects compressed cerebellum.10 Feeding arteries may be seen on CT angiography.
MRI
Early hemangioblastoma has prolonged T1 and T2 relaxation times and is indistinguishable from other masses. Later, it develops cystic components with adjacent flow voids. The cystic component has a high T2 signal equal to or higher than that of cerebrospinal fluid (CSF).11 An enhancing mural nodule is often near the pial surface. Vasogenic edema may be seen around the tumor. Gradient-recalled-echo imaging may demonstrate areas of susceptibility if there has been hemorrhage (Fig. 31-1).
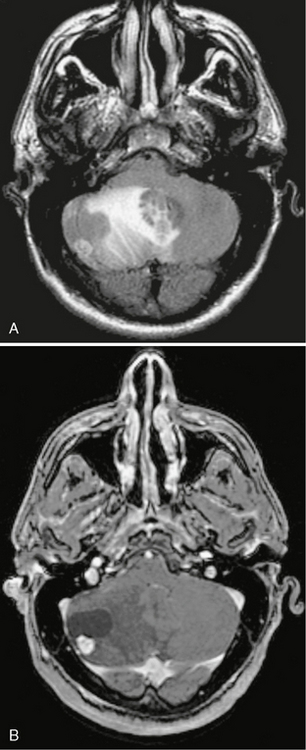
FIGURE 31-1 Hemangioblastoma. A, Axial FLAIR imaging demonstrates a cyst with a mural nodule in the right cerebellar hemisphere. Surrounding vasogenic edema and mass effect are present. B, Axial T1W post-gadolinium MR image demonstrates an avidly enhancing nodule with an associated cyst (same patient as in A). The nodule abuts the pia. The cyst does not enhance.
MR perfusion demonstrates increased cerebral blood volume with poor return to baseline (Fig. 31-2).
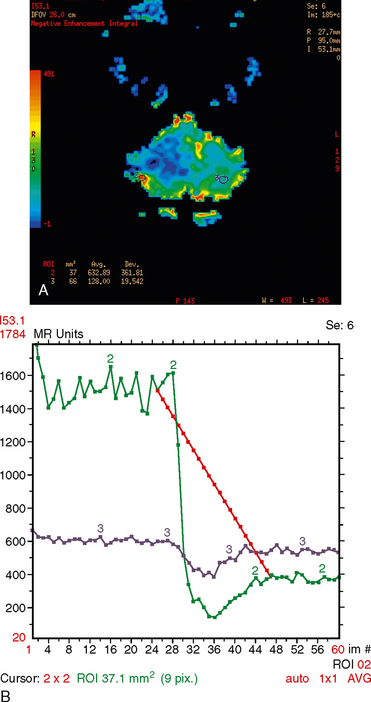
FIGURE 31-2 Hemangioblastoma perfusion. A, MR perfusion color map demonstrates increased blood volume in a region of solid tumor. B, Hemangioblastoma perfusion. MR perfusion cerebral blood volume graph demonstrates increased blood volume with poor return to baseline corresponding to the solid lesion in A.
Special Procedures
With angiography, the cyst is seen as an avascular mass. A mural nodule or solitary nodule is seen as a hypervascular mass with prolonged blush. Arteriovenous shunting may or may not be seen. Angiography may find hypervascular nodules not clearly seen on CT.10
 Hemangioblastoma is the most common primary intraparenchymal infratentorial tumor in adults and often presents as a solid and cystic mass, although purely solid and purely cystic lesions occur as well.
Hemangioblastoma is the most common primary intraparenchymal infratentorial tumor in adults and often presents as a solid and cystic mass, although purely solid and purely cystic lesions occur as well.
 An important differential factor in patients with von Hippel-Lindau disease is hemangioblastoma versus metastatic renal cell carcinoma.
An important differential factor in patients with von Hippel-Lindau disease is hemangioblastoma versus metastatic renal cell carcinoma.
 Differential diagnosis also includes juvenile pilocytic astrocytoma in younger patients (patients with hemangioblastoma are usually older unless they have von Hippel-Lindau disease).
Differential diagnosis also includes juvenile pilocytic astrocytoma in younger patients (patients with hemangioblastoma are usually older unless they have von Hippel-Lindau disease).
HEMANGIOPERICYTOMA
Hemangiopericytoma is a malignant tumor originating from the pericytes of Zimmerman around capillaries and postcapillary venules. The intracranial tumor arises from meningeal capillary pericytes and is also called an angioblastic meningioma. This tumor is also found in the skin and the musculoskeletal system.12
Epidemiology
There is a male predominance of 50% to 70%. The average age range at presentation is 38 to 42 years.
Clinical Presentation
Intracranial hypertension and headache are the most common features, but presentation may be related to location of tumor with motor and sensory deficits or seizures. Intracranial hemorrhage may also occur.13
There is a strong tendency for local recurrence and metastases outside the central nervous system (CNS) in comparison with meningioma. Hematogenous metastasis occurs to bone, lungs, and liver, in descending order of frequency.14 Metastasis also occurs to the kidney, pancreas, and adrenal glands.
Pathology
The intracranial location is similar to that of meningioma with approximately 15% of hemangiopericytomas found in the posterior fossa.16 Most tumors have dural attachments. There are also reports of tumors in sellar, suprasellar, and pineal regions.17 Very rarely they can be purely intraparenchymal.14
Imaging
Hemangiopericytoma can be indistinguishable from meningioma on imaging.
CT
Intracranial lesions are often heterogeneous, hyperdense, dural-based multilobulated lesions that, unlike meningioma, are not associated with calcifications (although rare tumor calcification has been reported) or hyperostosis and typically show heterogeneous enhancement. Low-density cystic and necrotic areas may be identified. Adjacent bone erosion is seen in greater than 50% of cases. Over half of hemangiopericytomas may have an associated dural tail.18
The lesion usually shows broad-based dural attachment; however, a narrow-based attachment favors hemangiopericytoma rather than classic meningioma.18
MRI
MRI features are also similar to those of meningioma. The lesion is often multilobulated and predominantly isointense to cortical gray on T1- (T1W) and T2-weighted (T2W) sequences. Enhancement is more heterogeneous than meningioma. Surrounding vasogenic edema may be mild to moderate (Fig. 31-3). More than half of hemangiopericytomas are associated with a dural tail. Prominent internal vascular flow voids may be present.18,19
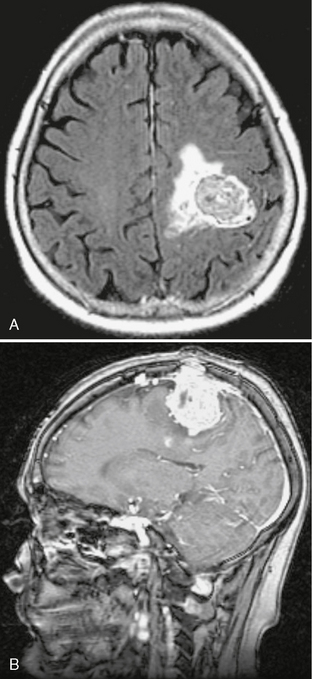
FIGURE 31-3 Hemangiopericytoma. A, Axial FLAIR demonstrates an isointense lesion with surrounding vasogenic edema in the left perirolandic region. B, Hemangiopericytoma. Sagittal T1W postcontrast MR image demonstrates a heterogeneously enhancing dural based mass (same patient as in A) with “mushrooming” into the parenchyma and erosion through the skull. There is an associated dural tail.
MR venography may demonstrate occlusion of dural sinuses by the mass. MR spectroscopy at short echo time may reveal a larger peak at 3.56 ppm secondary to higher levels of myoinositol, in contradistinction to meningioma.20 MR perfusion demonstrates increased cerebral blood volume with poor return to baseline, consistent with an extra-axial mass (Fig. 31-4).
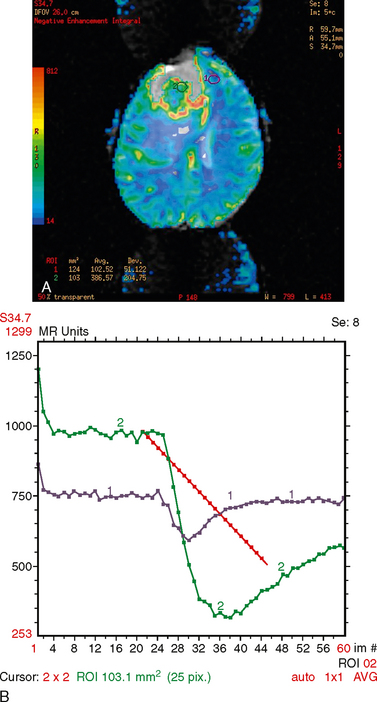
FIGURE 31-4 Hemangiopericytoma. A, MR perfusion color map demonstrates increased cerebral blood volume in the tumor, particularly around the periphery. B, MR perfusion cerebral blood volume graph demonstrates an increase in blood volume with poor return to baseline consistent with an extra-axial mass.
Special Procedures
The usefulness of angiography has been debated in the literature. Marc and colleagues considered the following angiographic features to be characteristic: dural arterial supply with few arterial feeders from which a myriad of small corkscrew vessels arise; dense tumor stain, slow circulation, and slow venous drainage.21 Alen and coworkers also found hemangiopericytoma to have a dense tumor stain with delayed venous drainage.14 Guthrie, however, reported that angiography was not helpful in the diagnosis.22
PRIMARY CNS LYMPHOMA
Epidemiology
The prevalence of primary CNS lymphoma substantially increased 2 decades ago but has only slightly increased in the past decade and currently represents up to 7% of intracranial tumors.23,24 The past increase was partly secondary to the AIDS epidemic as well as immunosuppression in solid organ transplant recipients. There was also a threefold increase in primary CNS lymphoma in immunocompetent patients, an increase that was independent of improved imaging and detection. This finding was thought to be related to a fundamental biologic change in the disease, because the intermediate grade of primary CNS lymphoma was not seen after 1983. The tumor appears to have become more histologically aggressive.24
Secondary lymphoma involving the CNS occurs less often since the advent of effective chemotherapy and, when present, affects the dura mater and leptomeninges more often than brain parenchyma. Hodgkin’s lymphoma rarely affects the CNS, and, if so, it does so late in the disease.25
Clinical Presentation
Primary CNS lymphoma presents as nonspecific neurologic findings, including focal symptoms, personality and cognitive changes, headaches, nausea, and vomiting. Symptoms are related to the size and location of the intracranial mass or masses. It may be preceded by a neurologic prodrome that may be misdiagnosed as multiple sclerosis.23
Pathophysiology
Primary CSN lymphoma is a densely cellular tumor with a distinct affinity for perivascular extension and predilection for the periventricular region.26 Diffuse microscopic disease is almost always present, which accounts for the ability of this tumor to produce distant disease and local recurrences.
Virtually all of these tumors are B-cell lymphomas (98%). The exact etiology is unknown because the brain has no endogenous lymphoid tissue.27 It is generally agreed that CNS lymphoma and systemic extracerebral NHL share the same cell of origin. There are two theories as to the origin of this tumor: (1) lymphocytes are attracted to the CNS by infection or inflammation and there undergo a transformation event, and (2) B lymphocytes that are already carrying a CNS-specific binding marker are activated, proliferate, and undergo neoplastic transformation.
There appears to be an association with infectious agents. Epstein-Barr virus genetic material has been found in over 90% of cases in immunocompromised patients.23 Cytomegalovirus has also been associated.
Pathology
Most lesions are supratentorial (75%) and have a predilection for the cerebral hemispheres, followed by basal ganglia, corpus callosum, and cerebellum. Leptomeningeal involvement occurs in about 12% of cases. Dural involvement is rare in primary CNS lymphoma. One percent of tumors occur in the spinal cord.23,24,27
Primary CNS lymphoma is frequently surrounded by edema, commonly spreads to leptomeninges and subpial regions, and is multifocal in almost 50% of cases. Focal necrosis and hemorrhage are common.25
Histology demonstrates monotonous, closely packed blue cells with a high degree of cellularity. Infiltrates are seen far beyond the borders of the grossly observed mass. Necrosis and hemorrhage are much more frequent in the lesions of immunocompromised patients.26
Neoplastic cells tend to cluster along vascular channels, a phenomenon that supports the theory that CNS lymphoma spreads diffusely through the brain by way of the perivascular spaces.26 A vasculitis-like appearance on histopathology may be seen because of tumor infiltrating the blood vessel walls. This angiocentric growth pattern—tumor cells forming multiple, thick layers around the host vessels and widening of the perivascular space—is one of the histopathologic hallmarks of the disease.
In general, lymphomas are divided into low-grade, highgrade, and possibly intermediate-grade NHL. No intermediate-grade cases have been reported since 1983. Today almost half of CNS lymphomas are of the high-grade, large-cell, immunoblastic subtype.24
The CSF may show elevated protein and decreased glucose levels. Cytology will often be negative.
Imaging
CT
Imaging features depend on the immune status of the patient. In an immunocompetent patient, primary CNS lymphoma appears hyperdense on noncontrast CT and demonstrates solid homogeneous enhancement. In immunocompromised patients it tends to be multicentric, hypoattenuated on unenhanced CT, and characterized by ring enhancement on contrast-enhanced CT.26,28,29
One of more characteristic features of primary CNS lymphoma is the tendency to abut the ependyma, the meninges, or both.30 This feature supports the theory that the lesion originated in the periadventitial cells of penetrating arterioles in the perivascular spaces.






