Moneeb Ehtesham • Imad S. Khan • J Mocco
Central nervous system arteriovenous malformations (AVMs) are congenital anomalies generally consisting of an abnormal tangle of thin-walled vessels (usually referred to as a nidus) located within the brain or spinal cord receiving inflow from one or more large- to medium-sized arterial pedicles with subsequent direct outflow to one or more large draining veins. Blood flowing through the nidus bypasses any normal intervening capillary bed, thereby creating a high-flow arteriovenous shunt which results in the presence of high-pressure arterialized blood in the draining veins.1 When located intracranially, this pathologic state presents a significant risk for devastating intracranial hemorrhage.2,3 Additional symptomatology can include seizures as well as cognitive or objective neurologic deficits related to either ischemia or venous hypertension and resultant cerebral or spinal cord edema.4 The primary goal of treating these lesions is to eliminate the risk of hemorrhage. Furthermore, treatment can successfully address the localized dysfunction created by presence of the nidus by means of decreasing seizure frequency and/or preserving or improving current neurologic function.5,6
There are four main treatment approaches toward managing a patient with an intracranial AVM: expectant observation, microsurgical resection, endovascular embolization, and radiotherapy.7 In reality, when pursuing treatment, many centers will use a combination of either embolization followed by surgery or embolization with subsequent radiotherapy. The evidence to support the efficacy of any specific combination of treatment modalities is currently limited to small retrospective series and remains a topic of controversy.8–10 Regardless, the fundamental approach to any treatment plan centers on appropriate patient selection followed by defining an optimal plan for treatment for each specific patient.
PATIENT SELECTION
There is tremendous controversy regarding specific patient populations in whom treatment of intracranial AVMs should be undertaken. This is evidenced by the recently halted ARUBA (A Randomized Trial of Unruptured Brain AVMs) trial, which was a prospective randomized multicenter trial designed to compare outcomes in patients with unruptured intracranial AVMs treated expectantly with medical management versus surgical and/or endovascular intervention.11–13 Early data reporting seems to indicate a significantly higher adverse event rate in the surgical/endovascular treatment group that led to early suspension of the study by the data safety monitoring board.14 The full results of this study are still pending and will provide important insight into appropriate patient selection in patients who have not had a prior hemorrhage. In contrast, there is considerable (although not universal) agreement that patients who present with an AVM-related hemorrhage are generally candidates in whom therapy should be considered. Data suggest that the rebleeding rate after AVM rupture is approximately 2% to 4% per year and that each hemorrhage episode carries with it a 30% to 50% morbidity rate as well as a 10% risk for mortality.15 Seizure control in patients with AVMs that have remained refractory to medical management can also be improved with treatment of AVMs.16
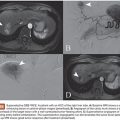
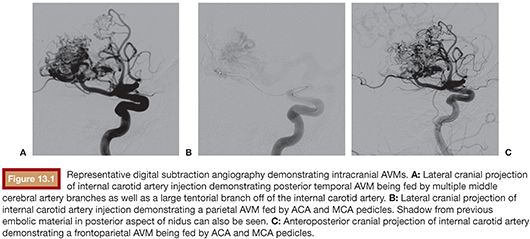
Assessment of the lesion’s angioarchitecture forms a second critical arm when assessing a patient as part of the treatment planning process. To this end, it is highly recommended that all patients with newly diagnosed intracranial AVMs undergo a comprehensive cervicocerebral digital subtraction angiogram as this allows for clear delineation of the vascular anatomy of the AVM as well as its flow dynamics. The key aspects of an AVM’s vascular anatomy include identifying all contributing arterial pedicles, draining veins, any associated aneurysms, as well as collateral circulation and any compensatory flow dynamics that are responsible for perfusing normal neural tissue1 (Fig. 13.1). Furthermore, from the specific regard of suitability for endovascular treatment, it is also important to delineate potential impediments to accessing the nidus starting from aortic arch disease, carotid bifurcation disease, tortuous extracranial or intracranial carotid anatomy, as well as tortuous feeding pedicles. It is also critical to determine whether arterial feeders to the nidus are either “end pedicles” exclusively perfusing the lesion or, instead, are “en passage” with a component of supply to normal neural tissue. In the latter situation, endovascular embolization without sufficient superselective catheterization can significantly jeopardize unaffected tissue and present a prohibitive risk for treatment. Up to 50% of AVMs are associated with aneurysms.17 These may either be intranidal or present on feeding arterial pedicles (flow-related). It is generally recognized that the presence of such aneurysms presents a higher risk of hemorrhage. More specifically, in patients presenting with hemorrhage, these aneurysmal lesions require urgent treatment if there is any suspicion based on anatomical distribution of the hemorrhage that they may be the contributory lesion.18,19 Finally, the flow dynamics through the lesion also presents important considerations for treatment planning especially with regard to endovascular therapy. Specifically, high-flow AVMs may harbor fistulous connections between feeding arteries and draining veins, and it is important to recognize these as their extent will affect the choice of embolic material used and whether specific pedicles can be safely targeted for embolization or not.
GOALS OF ENDOVASCULAR EMBOLIZATION
As detailed earlier, endovascular therapy represents only one of several therapeutic modalities available for treating intracranial AVMs.20 It is therefore critical that before deciding on a final treatment plan, a patient’s history and angiographic anatomy (as delineated by a digital subtraction angiogram) should be reviewed by a multidisciplinary team consisting of neurointerventionalists, neurosurgeons, and radiation oncologists to allow for formulation of an optimal course of therapy best suited to that particular patient. In this regard, endovascular embolization can be incorporated as a potent tool to either decrease flow to an AVM as a precursor to planned surgical resection or as a means of decreasing nidus size to allow for more focused stereotactic radiation. Rarely, and usually only in cases of small nidal size, can embolization be used as a solitary curative modality. Therefore, the goals of embolization will be determined by which additional treatment methodologies are to be employed for that particular lesion. In the setting of preoperative embolization, the ideal embolization strategy centers on decreasing inflow into the nidus from the aspect of the lesion that will be most difficult to access from the planned surgical corridor. In this setting, a focused approach targeting catheterization via deep arterial pedicles that will not be directly visible to the surgeon can result in improved intraoperative visibility during microsurgical dissection and decreased blood loss. Similarly, nidal penetration of embolic material helps to better delineate the nidus during microsurgical dissection, and knowledge of the angiographic pattern of embolization helps orient the surgeon particularly in the setting of large, complex nidal resections.21 In certain situations, surgical resection may not be feasible given the presence of eloquent neural tissue in close proximity to the nidus. In these scenarios, targeted embolization of nidal segments adjacent to eloquent regions can help decrease radiation exposure to important functional neural tissue as stereotactic radiotherapy can be targeted to the residual lesion with increased dose step-off between the radiation field and adjacent eloquent tissue. If pursued, endovascular embolization can be performed in a “staged” technique wherein specific portions of the nidus are targeted (frequently via a distinct single feeding pedicle at each sitting). When used, staged embolizations are felt to be safer as they decrease the total radiation exposure in a single sitting and also minimize the likelihood of overaggressive nidal embolization.22
TECHNIQUES
Materials
Various materials have been used for embolotherapy in the setting of endovascular AVM treatment. Of historical interest is the previous use of liquid coils (essentially deliverable soft/coiled wires), detachable balloons for proximal arterial pedicle occlusion, silastic pellets, silk sutures, and absolute alcohol. The use of these diverse agents has now largely been supplanted by the development of new liquid embolic agents. Key among these is the cyanoacrylate polymer, Histoacryl Blue (N-butyl cyanoacrylate [NBCA]; Aesculap, Center Valley, Pennsylvania), and the DMSO-based liquid precipitate, Onyx (Covidien, Irvine, California).20 NBCA is mixed with low-viscosity oil-based contrast medium (typically Ethiodol), and this mixture can be additionally supplemented with tantalum particles to enhance radiopacity. Given that NBCA precipitates nearly immediately upon contact with ionic solutes in blood, the delivery microcatheter has to be prerinsed with dextrose–water before administration. The flow rate and eventual penetration potential of the embolic agent as it emerges from the delivery catheter can be modulated by adjusting final viscosity based on concentration of Ethiodol as well as by adjusting the intensity and pace of the hand injection through the microcatheter. Given the significant adhesive properties of NBCA, it is imperative that the delivery catheter be removed immediately following delivery of agent as failure to do so can result in significant vascular injury as removal of a stuck microcatheter is attempted in a delicate intracranial vascular tree. Onyx is a newer liquid embolic agent that does not instantly precipitate, thereby theoretically allowing for greater downstream penetration. Furthermore, a developing Onyx cast does not demonstrate adhesion to the delivery catheter. As such, although it remains important to not allow excessive reflux along the microcatheter of Onyx precipitate, it is possible to build a small Onyx plug along the distal-most end of the microcatheter. This allows for enhanced proximal occlusion of the microcatheterized feeding pedicle, creating a “back pressure” that then permits subsequent Onyx injections to penetrate deep into downstream nidus.23,24 Although a treatment session with Onyx can take significantly longer as compared to when using NBCA, in our experience, it allows for more controlled and targeted delivery of embolic agent.
Patient Preparation
The initial aspects of patient preparation include a detailed discussion with the patient and/or family regarding the goals of treatment. As discussed earlier, it is our recommendation that all patients being considered for treatment be initially evaluated with a digital subtraction cervicocerebral diagnostic angiogram. Review of the data provided by this study and correlation of the findings with each patient’s specific clinical situation will allow the practitioner to formulate and present a comprehensive rationale for treatment. Following this, all patients should undergo an appropriate preoperative medical workup, including assessments of their medical comorbidities. The presence of significant medical conditions can significantly affect prognosis, and this should factor into discussions regarding the risks and benefits of therapy. Finally, renal function should be assessed as endovascular therapy of these lesions will often require a significant contrast load and those with impaired renal function should undergo appropriate preprocedure hydration. Following these steps, patients are brought to the angiography suite and placed under general anesthesia. Appropriate blood pressure monitoring in the form of invasive arterial transduction is established to allow for close blood pressure monitoring and titration.
Endovascular Treatment Platform
Arterial access for endovascular therapy is typically obtained via the transfemoral route and a 6-Fr short sheath. Alternative access can be established via a transradial route (if the aortic arch is significantly diseased or if a tortuous vertebral artery with unfavorable proximal anatomy is to be accessed). A longer sheath with its tip in the common carotid artery can be used if additional support is desired in the setting of a capacious descending aorta or aortic arch. In the absence of any recent intracranial hemorrhage, a loading bolus of 3,000 to 5,000 International Units of intravenous (IV) heparin is given and an activated clotting time goal of 200 to 260 seconds is set. If planning to use Onyx for embolization, it is imperative to use a DMSO-compatible microcatheter. Our current catheter of choice is the Marathon flow-directed catheter (Covidien, Irvine, California). Given the supple nature of this embolic delivery catheter, it is imperative to use an intermediate coaxial catheter system that will allow for distal navigation of the delivery microcatheter. For this purpose, we generally use a 6-Fr guide catheter (Neuron 0.70 in [Penumbra, Alameda, California] or Envoy MPD 0.70 in [DePuy Orthopaedics, Inc., Warsaw, Indiana]) along with a distal access catheter (DAC 0.38 in [Concentric Medical, Hertogenbosch, the Netherlands]). For the anterior cerebral circulation, the DAC is typically delivered into the proximal middle cerebral artery (MCA) or anterior cerebral artery (ACA), whereas for the posterior circulation, it is typically taken up to the level of the distal vertebral artery. Following this, the Marathon microcatheter is typically navigated over a 0.10-in microwire into a feeding arterial pedicle from which point onward the microwire is withdrawn into the microcatheter and the catheter tip is flow-directed to a point just proximal to entry into the nidus. The ability to navigate as close as possible to the nidus will allow for optimal penetration of delivered embolic material into the AVM nidus. This is critically important as embolization of feeding pedicles alone will not result in adequate treatment of the lesion as deafferented nidus will re-recruit additional arterial supply over time. Penetration of embolic material with resulting obliteration of the nidal lumen is critical for effective treatment of these lesions. It is therefore imperative that adequate effort be undertaken to navigate the microcatheter tip into as optimal a position as possible. The use of intermediate coaxial guide and delivery catheters (as detailed earlier) serves to assist with distal delivery of the microcatheter tip, particularly in the setting of tortuous vascular anatomy.
Embolization Technique
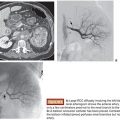
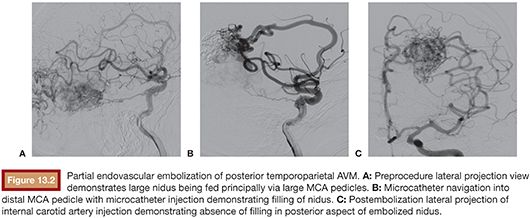
Once microcatheter delivery to an optimal location has been achieved, it is critical to study the relevant angiographic runs carefully before commencing embolization. These should consist first of an overall angiographic image of the vascular architecture of the relevant part of the brain, which is typically obtained by contrast injection into an upstream guide catheter. This should allow for complete visualization of the nidus and delineate all significant feeding arterial pedicles, draining veins, and blood flow patterns to the surrounding brain. Second, a microinjection through the microcatheter should be performed, and this will provide a focused overview of the angiographic anatomy downstream of the microcatheter tip.23 Particular attention should be paid to the nidal penetration of injected contrast material, the presence of any en passage arterial supply to normal brain, and the location and timing of venous outflow. Only upon obtaining a clear understanding of the anatomy and flow dynamics of the lesion should embolization be undertaken. In preparation for use of Onyx, the microcatheter is prerinsed in situ with an appropriate volume of DMSO. This volume will be equivalent to the microcatheter dead space volume, which is available in the microcatheter package. It is imperative that the catheter be purged of ionic contents before Onyx injection as failure to do so will result in Onyx precipitation within the catheter itself. However, it is also important to note that DMSO injection into the catheter should be performed very slowly as this solvent is caustic and can induce vasospasm and angionecrosis in downstream vasculature. Once this has been performed, Onyx liquid embolic is gently injected under direct fluoroscopic visualization, and careful modulation of the hand injection intensity and pace is used to allow the injected stream to gradually progress down the catheterized arterial pedicle and into the nidus. It is essential that Onyx penetration into the draining venous system be avoided as venous compromise significantly increases the risk of hemorrhage and must be avoided. As the Onyx cast builds up proximally to the catheter tip, the initial injection is halted and the intravascular embolic cast is allowed to solidify. Subsequent injections are modulated very carefully to maximize distal penetration of embolic material and minimize proximal reflux. Although a small degree of Onyx reflux along the distal catheter can be tolerated, a significant cast should not be created along the microcatheter as this will impede its removal. Even though Onyx is not adhesive, its cohesive properties can cause microcatheters to get lodged, and attempted removal can result in significant traction on the intracranial vascular tree, significantly increasing the risk of hemorrhagic complications.25,26 Once embolization through the pedicle of choice is completed, the microcatheter is gently disengaged from the Onyx cast. This will frequently require the progressive building of tension on the microcatheter tip. As this tension is built, the intermediate DAC catheter that was previously parked in the proximal vasculature can be brought into the proximal portion of the feeding pedicle, allowing for more direct force transmission to the microcatheter tip and less distortion of the intracranial vascular anatomy. Upon successful disengagement, the microcatheter is removed. Follow-up angiography is performed to assess the results of the embolization (Fig. 13.2). At this point, it is critical to look specifically for any signs of untoward occurrence including compromise of venous outflow, presence of embolic material in unintended locations, or any extravasation indicative of hemorrhage. In the absence of these conditions, a new microcatheter can then be navigated into the vascular tree for purposes of microcatheterizing additional feeding arterial pedicles if desired. Although there is debate regarding how much of the nidus can be safely embolized in a single treatment setting, in our practice, we generally limit ourselves to approximately one-third of the lesion. Embolizations that are more aggressive than this carry with them the risk of increasing hemorrhagic complications resulting from significantly increased transient flow through the remaining nonembolized nidus.25,27 Therefore, it is important to emphasize tight blood pressure control following embolization. If there is inadvertent embolization of a significantly larger portion of nidus than was initially intended or any untoward sign concerning for venous outflow compromise, then strong consideration should be given to urgent same-day surgical resection of the remaining lesion. In case staged embolizations are deemed suitable, the patient is brought back for another embolization session 2 to 3 weeks after the previous session.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree