|
Identify the role of diagnostic medical sonography in the assessment of abdominal vascular structures.
Perform sonographic evaluation of the abdominal vascular system.
Describe the patient preparation, equipment considerations, and scanning techniques and Doppler protocols for normal and abnormal abdominal vascular structures.
Identify circulatory anatomy, name the layers of blood vessels, and distinguish the difference between arteries and veins.
Recognize the sonographic appearance and relational anatomy of the abdominal vascular system.
Describe the pathology, etiology, clinical signs and symptoms, and sonographic appearance or aortic pathology to include atherosclerosis, aneurysms, dissection, rupture, inflammatory aneurysms, stenosis, and vascular insufficiency.
Discuss the complications of an aortic graft, including pseudoaneurysms, graft aneurysms, hematomas, abscesses, and occlusions.
Describe the pathology, etiology, clinical signs and symptoms, and the sonographic appearance for venous abnormalities, including vena caval obstruction, tumors, venous enlargement, thrombosis, aneurysm, hepatic venous abnormalities (Budd-Chiari syndrome), and portal venous abnormalities (portal thrombosis and portal venous hypertension).
Formulate a list of differential diagnosis based on correlating the patient’s clinical history, laboratory values, results of related diagnostic procedures, and the sonographic tissue characteristics.
Identify technically satisfactory and unsatisfactory sonographic examinations of the vascular system.
posterior, a short distance medial, and then superior to form the ascending aorta. It then curves lateral and posterior to form the aortic arch. As the aorta completes its curve at the arch, it begins to descend inferiorly into the chest. This portion, the descending aorta, soon gives rise to the thoracic aorta. Once the aorta penetrates the diaphragm, it is termed the abdominal aorta until it bifurcates into the common iliac arteries prior to entering the pelvic cavity. It is the abdominal aorta that is most accessible to sonographic examination (Fig. 6-2).
 FIGURE 6-1 Segments of the aorta. The common reference segments, ascending, arch, descending, thoracic, and abdominal aorta can be identified on the illustration. |
 FIGURE 6-2 Major branches. An anterior illustration of the abdominal aorta showing the anatomic location of its major branches. |
more comprehensive imaging of the iliac arteries is accomplished by placing the transducer in the iliac fossa and angling medially with the scan plane oriented approximately 45 degrees from midline. Demonstration of the length of the iliac arteries is thus achieved. At times, successful imaging of the iliac arteries requires a distended urinary bladder. In this case, the transducer is placed in the midline of the pelvis and oriented 45 degrees from midline. Lateral angulation will result in visualization of the iliac vessels.
in which the IVC expands during this maneuver. With suspended inspiration, the IVC expands because of increased intrathoracic pressure and decreased blood flow into the heart. During the Valsalva maneuver, the IVC collapses because of the increased abdominal pressure associated with this technique.8,9
branch artery. Detection of a hemodynamically significant stenosis is discussed in detail later in this chapter.
 FIGURE 6-10 Relationships. Collective illustration of the major branches of the aorta, inferior vena cava, and the portal system helps visualize the abdominal vascular and organ relationships. |
TABLE 6-1 Relational Anatomy | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||
 FIGURE 6-12 Aneurysms. The illustration presents three types of true aneurysms: (A) saccular, (B) fusiform, circumferential, and (C) dissecting. |
in the evaluation of suspected aortic aneurysm and can be used to monitor the growth of aneurysms over time.23 However, there are some important considerations to keep in mind to avoid misdiagnosis of an aneurysm. Tortuosity may make the aortic diameter appear larger than it is. This occurs when the plane of imaging is not truly perpendicular to the aortic walls. Therefore, careful observations should be made of the aortic curvature in these instances to avoid misrepresentation of a tortuous aortic segment as an aortic aneurysm. Excessive air in the abdomen or obesity may obscure the distal aorta and iliac vessels and render some aneurysms invisible. Lymphadenopathy may also confound the picture.21,22
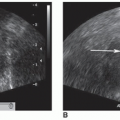
organs and structures.12 Other findings suggestive of aortic aneurysm rupture include irregular intra-abdominal fluid collections in association with aortic aneurysm and diffuse irregular hypoechoic areas near an aortic aneurysm.
aneurysms to rupture, early detection is important so that prompt treatment can be obtained.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree