Benign vascular tumors
Hemangiomas of subcutaneous/deep soft tissue
Capillary
Cavernous
Arteriovenous
Venous
Intramuscular
Synovial
Epithelioid hemangioma
Angiomatosis
Lymphangioma
Vascular tumors of intermediate malignancy (locally aggressive)
Kaposiform hemangioendothelioma
Vascular tumors of intermediate malignancy (rarely metastasizing)
Retiform hemangioendothelioma
Papillary intralymphatic angioendothelioma
Composite hemangioendothelioma
Kaposi’s sarcoma
Malignant vascular tumors
Epithelioid hemangioendothelioma
Angiosarcoma
16.2.2 ISSVA Classification
In 1982, Mulliken and Glowacki [8] proposed a useful classification for vascular anomalies, which was then adopted by the International Society for the Study of Vascular Anomalies (ISSVA) in 1996 and updated in 2014 [6]. This classification is based on the cellular turnover, histologic features, natural history, and physical findings of the vascular anomalies [8]. Lesions are divided into tumors (neoplastic growth of vascular endothelial cells) and vascular malformations (vascular structural anomalies with normal endothelial turnover) [6, 8, 9] (Table 16.2).
Table 16.2
ISSVA classification of vascular tumors and malformations
Tumors |
Benign |
Infantile hemangioma |
Congenital hemangioma (RICH, NICH, PICH)a |
Others: tufted angioma, spindle cell hemangioma, epithelioid hemangioma, pyogenic granuloma |
Locally aggressive or borderline |
Kaposiform hemangioendothelioma, retiform hemangioendothelioma |
Papillary intralymphatic angioendothelioma (PILA), Dabska tumor |
Composite hemangioendothelioma, Kaposi’s sarcoma, others |
Malignant |
Angiosarcoma |
Epithelioid hemangioendothelioma |
Malformations |
Simple (i.e., venous, lymphatic, capillary, and arterial) |
Combined: defined as two or more vascular malformations found in one lesion |
Associated with other syndromes: Klippel-Trenaunay syndrome, Parkes Weber syndrome, etc. |
The use of this classification system has been strongly recommended in recent years because of its effectiveness and usefulness for determining the appropriate treatment in patients with vascular lesions, particularly in pediatric patients where vascular lesions represent the most common cause of soft tissue lesion [10]. Therefore, we will use this classification in this chapter; however, Table 16.3 [4, 11–13] indicates comparison between the two classification systems.
Table 16.3
Equivalence in terminology between the two main classification systems for vascular lesions
WHO classification | ISSVA |
|---|---|
Cavernous hemangioma | Venous malformation |
Venous hemangioma | Venous malformation |
Intramuscular hemangioma | Venous malformation (mainly) |
Lymphangioma | Lymphatic malformation (localized) |
Lymphangiomatosis | Lymphatic malformation (diffuse) |
Arteriovenous hemangioma | Arteriovenous malformation |
Capillary hemangioma | Infantile hemangioma |
Other entities, such as glomus tumors and synovial hemangiomas, will be discussed separately.
16.3 Vascular Tumors
16.3.1 Benign Vascular
We will only describe the most common lesions and those associated with clinical or specific nosological entities.
16.3.1.1 Infantile Hemangioma (IH)
Infantile hemangiomas are the most common vascular tumor of infancy [10]. They occur three to five times more frequently in females compared to males [14] and may be present at birth but are frequently diagnosed by 3 months of age [10]. In most cases, diagnosis is made clinically through the observation of a subcutaneous bluish red mass that looks like the surface of a strawberry (Fig. 16.1).


Fig. 16.1
Proliferative infantile hemangioma in a 15-month-old boy. Clinical photograph shows a diffuse and lobulated mass in the arm with superficial involvement, which causes its strawberry-like appearance
These tumors undergo two biologic phases:
The proliferative phase, which is characterized by rapid endothelial growth in the first few months of life that stabilizes in size at approximately 9–10 months of age. Reflecting the characteristic high-flow component of this phase, the tumor manifests as a pulsatile and warm mass [15].
On pathological examination, whatever the phase, IHs express a unique immunophenotype (glucose transporter protein-1 (GLUT1)), which differentiates them from other vascular lesions [18].
Infantile hemangiomas are most commonly seen on the face and neck (60 % of cases), followed by the trunk (25 %) and extremities (15 %) [19]. They are multifocal in approximately 30 % of the cases [1].
During the proliferative phase, the lesion appears as a well-defined mass with variable echogenicity. The vessels may be visible using US. Doppler demonstrates a characteristic hypervascular lesion with a high density of vessels (arteries and veins) and a low resistance on spectral analysis (Fig. 16.2) [2, 20]. Despite the high-flow nature of the lesion during this phase, there is a distinct soft tissue lesion that usually contains a single afferent artery and no direct arteriovenous shunting, unlike arteriovenous malformations [2]. During the involuting phase, IHs appear hyperechoic with a low density of vessels.


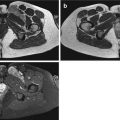
Fig. 16.2
Infantile hemangioma. Nine-month-old boy with subcutaneous mass in his posterior cervical neck. (a) Axial Ultrasound. (b) Axial color Doppler ultrasound. (c) Spectral analysis obtained in tumor center. (d) Axial T1-weighted MR image. (e) Axial T2-weighted MR image, with fat suppression. (f) Axial T1-weighted MR image after Gadolinium contrast administration with fat suppression. (a–c) Ultrasound shows a solid mass with well-defined margins in the subcutaneous soft tissues of the neck (a). The lesion is predominantly hyperechoic with scattered hypoechoic foci that correspond to vessels (white arrow). (b, c) Color Doppler shows hypervascular lesion with low-resistance arteries. (d–f) MRI shows a well-defined, lobulated soft-tissue mass confined to the subcutaneous soft tissues. The mass is isointense relative to muscle on the unenhanced T1-weighted image (d), hyperintense on T2-weighted image (e), and shows uniform enhancement (f). All three images demonstrate small, intralesional signal void foci (black arrows) due to fast flow vessels. There is no invasion of the underlying muscle and no perilesional oedema
The appearance of IHs using MR imaging is indicative of their biologic phase. The proliferative phase is characterized by a well-circumscribed lobulated lesion with low or iso-signal intensity on the T1-weighted images and a high signal intensity on the T2-weighted images; the IH lesions also show an early and homogeneous enhancement after gadolinium administration (Fig. 16.2) [21]. Fast-flow vessels appearing as flow voids can be depicted within and around the lesion [2]. During the involuting phase, the increasing amount of fat within the tumor increases the signal intensity of the lesion on the T1-weighted images, which is associated with less avid contrast enhancement (Fig. 16.3).


Fig. 16.3
Infantile haemangioma of the cheek in a 7-year-old girl during involution phase. (a) Axial T1-weighted MR image. (b) Axial T2-weighted MR image with fat suppression. (c) Axial T1-weighted MR image after Gadolinium contrast administration with fat suppression. MRI images show a subcutaneous hyperintense mass on T1-weighted image (a), slightly hyperintense on T2-weighted image (b) with minimal enhancement (c). No flow voids are seen
The presence of a high resistance index on the spectral analysis or a marked perilesional edema on the T2-weighted images is suggestive of other tumoral lesions, such as sarcomas, neuroblastomas, myofibromatosis, tuft hemangiomas, metastatic neuroblastomas, or other tumors [2].
Multiple hemangiomas of the skin are usually linked with visceral hemangiomas. Segmental hemangiomas may be associated with PHACE syndrome (see Sect. 16.6.1) [22]. Most of these segmental hemangiomas are telangiectatic or reticular and do not demonstrate a high-flow pattern on Doppler US.
In the majority of the cases, no treatment is required due to spontaneous involution. However, 10–20 % of the cases require treatment, including cases with periocular location with vision compromise, high-output cardiac failure, ulceration, compression of the airway, facial hemangiomas with rapid growth and distortion, and symptomatic muscular hemangiomas. Medical treatment is usually attempted first, and propranolol is typically the first-line therapy with excellent results in most cases [23]. Other treatments include corticosteroids, vincristine, interferon, and laser therapy. Surgery is required when medical alternatives are ineffective, mostly in cases involving function-threatening, life-endangering, and disfiguring lesions [16].
Key Points
Infantile hemangioma.
The most common vascular tumor of infancy.
Characterized by two biologic phases (proliferative/involuting).
Positive for the GLUT1 marker at both stages.
Presence of a high-flow soft tissue mass.
If perilesional edema is present, other tumoral lesions must be ruled out.
16.3.1.2 Congenital Hemangioma (CH)
CHs are rare and fully developed at birth; therefore, they are potentially seen in utero. Three subtypes have been identified [6, 24, 25]:
Rapidly involuting congenital hemangiomas (RICH), which completely regress during the first 2 years of life.
Noninvoluting congenital hemangiomas (NICH), which demonstrate growth proportional to that of the child without regression (Fig. 16.4).

Fig. 16.4
Congential hemangioma in a 3-year-old boy. (a) Clinical photograph. (b) Axial color Doppler image with spectral display obtained in tumor center. (a) Non-involuting congenital hemangioma (NICH) is seen as an overlying bluish discoloration with clear peripheral halo (arrow). The lesion has been present since birth, growing proportionally to the patient’s growth. (b) Color Doppler image shows the marked increased vascularity inside the lesion with low-resistance arteries. The imaging features are indistinguishable from those of infantile hemangioma
Partially involuting congenital hemangiomas (PICH), which have a distinct behavior, evolving from RICH to NICH-like lesions. RICH may be clinically difficult to differentiate from infantile hemangioma but the GLUT1 marker is negative.
Congenital hemangiomas share the same imaging findings on sonography. These features are similar to those of infantile hemangiomas, except for the presence of intravascular thrombi, vascular aneurysms, and arteriovenous shunting [26].
NICH are usually treated by a surgical resection [27].
16.3.1.3 Epithelioid Hemangioma
This benign vascular tumor is encountered in adult patients with a variable sex predilection [5, 28]. It is usually superficially located and is responsible for red nodules mainly on the face and fingers. Furthermore, deep locations, including in the bone, are possible [5]. Epithelioid hemangiomas are differentiated from Kimura’s disease, a chronic inflammatory lesion affecting young Asian men, which shares clinical and pathological similarities to epithelioid hemangiomas [29]. The imaging is nonspecific [28].
16.3.2 Intermediate and Malignant Vascular Tumors
16.3.2.1 Kaposiform Hemangioendothelioma (KHE)
KHEs are locally aggressive, rare vascular tumors of intermediate malignancy [5, 6]. Their pathology is characterized by frequent lymphatic abnormalities [10]. Even if the tumor does not metastasize, intra-abdominal deep forms have a poor prognosis because they are rarely curable by surgery [30].
MR imaging helps to differentiate KHEs from infantile hemangiomas. KHE appears as an ill-defined lesion with hemosiderin deposits and smaller feeding and draining vessels. Moreover, KHEs involve multiple tissue planes and destruction of the adjacent bones is also observed [10, 15].
16.3.2.2 Kaposi’s Sarcoma
Kaposi’s sarcoma (KS), also known as KS-associated herpesvirus (KSHV), is a locally aggressive vascular tumor associated with the human herpesvirus-8 (HHV-8). This tumor is probably of lymphatic origin and is characterized by neoangiogenesis and proliferation of spindle-shaped cells with inflammation and edema [33]. It is usually a multicentric disease that originates in the lymphoreticular system and may involve the skin, lymph nodes, lungs, gastrointestinal tract, liver, spleen, and musculoskeletal system. Four types of KSs have been described: classic KS (chronic form, patients older than 60 years old), endemic KS (middle-aged adults and children, African KS), KS in iatrogenically immunosuppressed patients (related to solid organ transplantation or immunosuppressive therapy), and AIDS-related KS [28, 33]. The topography of the lesions and their aggressivity depend on the type of KS. For example, KS AIDS is often aggressive and multifocal (face, genitals, lung, lower extremities). Imaging depends on the location of the lesion and the lymph nodes are typically hypervascular (Fig. 16.5) [28, 33].


Fig. 16.5
Kaposi sarcoma in a 35-year-old man (endemic type). Computed tomography after iodinated-contrast injection shows an enhancing bilateral inguinal lymphadenopathy (arrows)
16.3.2.3 Epithelioid Hemangioendothelioma (EH)
This rare malignant vascular tumor demonstrates a local recurrence rate of 10–15 % and a high metastatic rate (20–30 %). About half of the cases are multifocal [34, 35]. This lesion can arise in any vascular tissue and has been reported to be located on the skin, muscle, vasculature, bone, brain, and stomach. Tumor location in the liver and lungs may be confused with metastatic disease [35–37]. The adjacent vessels may be thrombosed, which may lead to symptoms. Calcifications can be observed [36, 38]. This lesion is often associated with Kasabach-Merritt syndrome.
16.3.2.4 Angiosarcoma
Angiosarcoma is a high-grade malignant tumor with a high mortality rate [39]. It may involve the skin (33 % of the cases), the deeper soft tissues (24 % of the cases), the internal organs, or the bones [38]. The presence of chronic lymphedema (Stewart-Treves syndrome) occurs in approximately 10 % of the cases and is a well-known risk factor for angiosarcoma [38]. Stewart-Treves syndrome is usually reported in the upper extremities after a mastectomy for breast cancer. Additionally, it can be seen in the lower extremities after hysterectomy for uterine cancer, trauma, or infection [40]. Angiosarcoma is rarely radiation-induced, and it exceptionally arises in vascular lesions [41]. Clinically, two types of angiosarcoma can be seen:
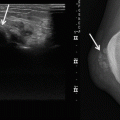
Metastasis most frequently involves the lung and lymph nodes, followed by the liver, the bones, and the soft tissues [39]. The imaging appearance depends on whether the lesion is located superficially or deep. Skin thickening or focal soft tissue nodules are observed when the lesion involves the skin and subcutaneous tissues. The imaging features are not specific and include intermediate echogenicity, variable signal intensity on T2-weighted images, and variable contrast enhancement (Fig. 16.6) [28]. When the lesion is associated with chronic lymphedema, extremity enlargement, diffuse skin thickening, and edema of the subcutaneous connective tissue can be seen (Fig. 16.7).

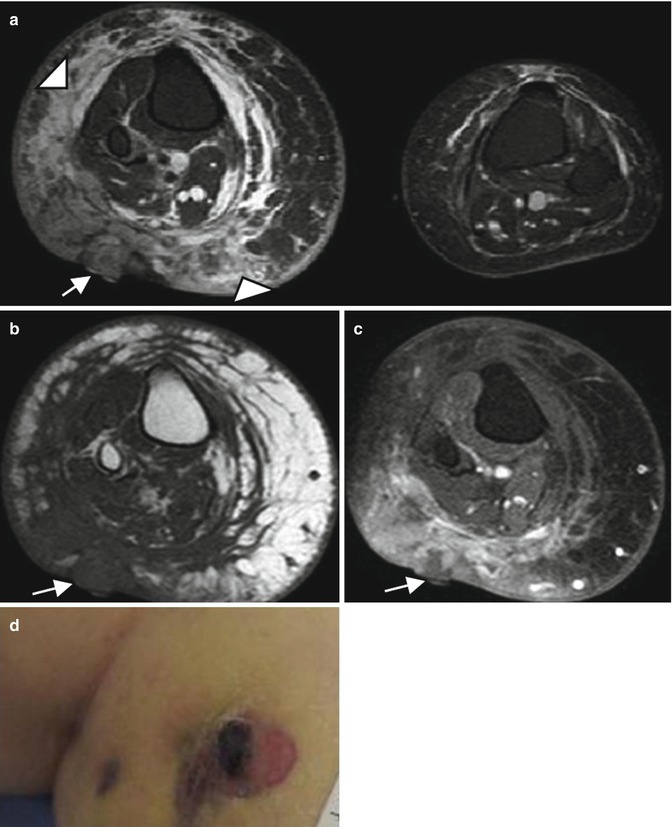

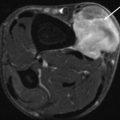
Fig. 16.6
Angiosarcoma in a 70-year-old men. (a) Clinical photograph. (b) Axial color Doppler image. (c) Computed tomography. (a) Clinical appearance of numerous skin lesions. (b) Ultrasound with color Doppler shows nodular superficial tissue mass, slightly hypoechogenic and predominately peripheral flow within the soft tissue mass. (c) Computed tomography demonstrates a non specific subcutaneous nodular lesion

Fig. 16.7
Stewart-Treves syndrome in a 66-year-old woman who was treated 20 years ago for uterus cervical cancer. (a) Axial T2-weighted MR image, with fat suppression. (b) Axial T1-weighted MR image. (c) Axial T1-weighted MR image after Gadolinium contrast administration, with fat suppression. (d) Clinical photograph. (a) Axial T2-W fat-suppressed MR images reveals an enlarged right extremity with subcutaneous edema (arrowheads) (a–c) Nodular mass of low signal intensity on T1, with low to intermediate and heterogeneous signal intensity on T2 and a heterogeneous enhancement (arrows) representing the angiosarcoma. (d) Clinical photograph shows the purplish skin lesion
16.4 Vascular Malformations
Vascular malformations are defined by an abnormal vasculogenesis with a normal turnover of endothelial cells. They are subcategorized according to their hemodynamics [42]:
Low-flow malformations (venous, lymphatic, capillary, capillary-venous, and capillary-lymphatic-venous)
High-flow malformations (arteriovenous malformations [AVMs] corresponding to an anastomosis between an artery and a vein through a nidus and arteriovenous fistulas [AVFs] with a direct anastomosis between a main artery and a main vein)
This distinction is important for planning treatment. Interventional radiologists play a major role in these lesions with the increasing use of sclerotherapy and embolization therapies.
16.4.1 Venous Malformations (VMs)
VMs represent the most common peripheral vascular malformation [43, 44]. They can be simple, combined (e.g., capillary-venous and capillary-lymphaticovenous malformations), or syndromic (associated with Klippel-Trenaunay syndrome, blue rubber bleb nevus syndrome, or Maffucci syndrome). They are present at birth, but patients usually develop symptoms during late childhood or early adulthood (especially during puberty and pregnancy). They vary in size and shape and can be localized and well defined or diffuse and infiltrative. They can involve the superficial and/or deep tissues. When superficial, patients typically demonstrate bluish skin abnormalities and a soft compressible and nonpulsatile soft tissue mass [45]. They typically expand during the Valsalva maneuver and decompress with extremity elevation and local compression [17, 45]. They can also be deep, involving many anatomical structures, including the muscle, synovial membrane, bone, and liver [4, 45, 46]. They may cause pain, impaired mobility, and skeletal deformities. They are usually located on the extremities (40 %), head and neck (40 %), and trunk (20 %) [17, 45]. Elevated D-dimer levels are a specific biomarker for VMs [47].
Pathologically, VMs are characterized by small- or large-sized venous channels connected with the normal venous system. They may contain thrombi whose dystrophic mineralization produces phleboliths [43]. Although pathologists now apply the new ISSVA nomenclature [46, 48], the historical terminology is kept in the current WHO classification (Table 16.1) [5]. Therefore, entities such as cavernous, venous, or intramuscular hemangiomas usually correspond to venous malformations and are still frequently used in pathologic reports using the WHO classification [5, 38, 46].
Phleboliths and dystrophic calcifications are easily detected on radiographs and CT and are highly suggestive of VMs (Fig. 16.8) [2]. In case of extensive malformations, the involvement of the adjacent joints or bones is possible, such as cortical erosion, periosteal reaction, regional osteopenia, and bony overgrowth [49, 50].


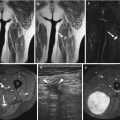
Fig. 16.8
Venous malformation of the left plantar region. (a) Plain radiograph. (b) Axial ultrasound. (c) Axial spin-echo T2-weighted MR image, with fat suppression. (d) Axial gradient-echo T2-weighted MR image. (a) Plain radiography demonstrates periosteal reaction, probably related to chronic vascular stasis (black arrowhead), and phlebolith (circle). This finding is characteristic of intramuscular venous malformation. (b) US shows hypoechogenic soft-tissue lesion with hyper echogenic foci with posterior acoustic shadowing corresponding to phleboliths (arrow). (c, d) Spin-echo and gradient-echo T2-weighted images show the VM as multiple slightly hyperintense serpiginous channels (star) with rounded hypointense phleboliths (white arrowheads), extending within the plantar muscles
Ultrasound shows an extremely variable appearance of the lesion, ranging from predominantly solid to multicystic lesions [1]. VMs can be well defined or infiltrative. These usually hypoechoic and heterogeneous lesions consist of tubular vascular or cavity compressible areas, reflecting the presence of vessels. The phleboliths are usually seen as hyperechoic foci. After applying compression, the movement of blood into the cavities can be identified (Fig. 16.9). Doppler US typically shows no flow or low-velocity flow. In the absence of a spontaneous vascular signal, dynamic maneuvers such as the Valsalva maneuver or manual compression by the probe followed by its decompression can cause the appearance of vascular signal although thrombosis is possible.


Fig. 16.9
Five-year-old girl with intramuscular venous malformation. (a) Ultrasound image. (b) Ultrasound image after local compression. (c) Color Doppler image. (a) Ultrasound image shows a heterogeneous lesion (arrowhead) with internal fluid component (arrow). (b) The lesion is compressible (arrowhead). (c) After decompression, filling of the cavities (vessels) can be observed on color Doppler US
MRI is an excellent imaging modality to define the extent of the lesions and their relationship with the adjacent structures. VMs demonstrate well- or ill-defined limits, but they typically do not produce any significant soft tissue mass syndrome, which suggests diagnosis. Vascular vessels are seen as tubular and serpiginous cavities with hypo- to iso-signal intensity on T1-weighted images and strong hyper-signal intensity on T2-weighted images, separated by hyperechoic fat (Fig. 16.10). Fat-suppressed T2-weighted images are particularly useful to evaluate the extent of the VMs. Phleboliths appear as small low-signal-intensity foci on all pulse sequences. Sometimes fluid-fluid levels due to hemorrhage or high protein content can be seen (Fig. 16.11) [1], though they are less common compared to lymphatic malformations. Contrast-enhanced 3-D acquisitions after dynamic gadolinium administration should be performed to evaluate the perfusion of the malformation and its drainage into the venous system. VMs are characterized by a lack of arterial and early venous enhancements and the absence of enlarged feeding vessels or arteriovenous shunting. They typically demonstrate a slow and progressive enhancement. Characteristic nodular enhancement of tortuous vessels can be observed on delayed venous phase images (Fig. 16.12) [3, 51]. The time between the beginning and the maximal enhancement is between 50 and 100 s [52]. Less specific appearances may be encountered when the lesion is composed of small vessels (Fig. 16.13) or in the absence of adipose tissue [53]. Intramuscular VMs often demonstrate serpiginous venous channels between the muscle fibers that are oriented along the long axis of the involved muscle [1]. Intra-articular involvement may lead to chronic hemarthrosis and joint destruction [54, 55] (Fig. 16.14). Localized intravascular coagulopathy is more frequent in diffuse compared to focal VMs and can be responsible for pain and thrombosis [56].






Fig. 16.10
Ten-year-old boy with subcutaneaous venous malformation. (a) Axial T1-weighted MR image. (b) Axial T2-weighted MR image with fat suppression. (c) Axial T1-weighted MR image after Gadolinium contrast administration with fat suppression. (a) MR images show multiple serpiginous areas confined to the subcutaneous soft tissues, isointense on T1-weighted image, separated by hyperintense areas corresponding to fatty components. (b) The lesion is hyperintense on T2-weighted image and (c) shows diffuse enhancement

Fig. 16.11
Venous malformation with fluid-fluid level in a 5-year-old boy with recent trauma. Axial T2-weighted MR image with fat suppression reveals numerous intramuscular cavities with fluid–fluid levels. Note the presence of low signal intensity spot corresponding to phlebolith

Fig. 16.12
Intramuscular venous malformation of the upper extremity. (a) Sagittal T1-weighted MR image. (b) Sagittal T2-weighted image with fat saturation. (c) Maximum intensity projection (MIP) after 3D contrast-enhanced MR angiography, late venous phase. (a) T1-weighted image shows an intramuscular and extensive lesion which involves the forearm muscles and elbow joint, surrounded by fat component (arrowheads). (b) The lesion is composed of multiple tortuous hyperintense vessels on T2-weighted image representing a slow flow vascular malformation. At least two phleboliths (circles) are seen as low signal foci inside the dilated veins. (c) Late venous phase image from gadolinium-enhanced 3D MR angiography shows characteristic filing of cavernous spaces with diffuse and nodular enhancement (star)

Fig. 16.13
Intramuscular venous malformation of the thigh in a 32-year-old man with pain exacerbated during exercise. (a) Ultrasound. (b) Sagittal T1-weighted MR image. (c) Axial T1-weighted MR image after Gadolinium contrast administration, with fat suppression (a) Ultrasound shows a nonspecific intramuscular soft tissue lesion (white arrowhead) (b) MR image demonstrates fat component surrounding the lesion (black arrowheads), (c) and serpiginous enhancement after gadolinium contrast administration (arrow)

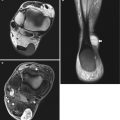
Fig. 16.14
Patient with diffuse and infiltrative venous malformation of the knee. (a) Plain radiograph. (b) Coronal T2-weighted image with fat saturation. (c) Axial T2-weighted image with fat saturation. (a) Plain radiograph shows a widening of intercondylar notch with erosion of the medial femoral condyle. (b, c) MRI shows infiltrative and serpentine vascular spaces with involvement of multiple compartments of the knee. Hyperintense nodular and serpentine areas of vascular vessels are seen in the subcutis (arrowheads), muscles and joint. Note the dilated deep veins (black arrowhead)
Most VMs are managed conservatively with a compression bandage of the extremity and medical antalgics for pain. In cases of major pain, joint involvement, and functional or cosmetic problems, the first-line treatment for VMs is sclerotherapy (dehydrated ethanol, sodium tetradecyl sulfate, polidocanol, and bleomycin), which can be followed by resection, laser therapy, and photodynamic therapy [57, 58].
Key Points
Venous malformations
Superficial or deep (muscle, synovial membrane, bone, liver) and localized or diffuse
Expansion during Valsalva maneuver/decompression with extremity elevation and local compression
Phleboliths +++
Tubular and serpiginous cavities strongly hyperintense on T2-weighted images
Diffuse and delayed enhancement of the slow-flowing venous channels after contrast administration
16.4.2 Lymphatic Malformations (LMs)
LMs represent the second most common type of vascular malformation after venous malformations [59]. These low-flow vascular malformations are classified into three types depending on the size of the cystic cavities [1, 2, 45, 60, 61]:
Macrocystic types (formerly cystic hygroma), with cysts >2 cm
Microcystic types (formerly lymphangioma), with cysts between 1 and 2 cm
Mixed types with capillary and/or venous malformations
Pathologically, they are composed of serpiginous dilated lymphatic channels that do not communicate with the normal lymphatic system [45], except in the retroperitoneum. Macrocystic and microcystic lesions are pathologically indistinguishable [61]. Diagnosis is suggested after a positive test for markers of lymph vessels, such as the vascular endothelial growth factor receptor 3 (VEGFR-3), LYVE-1, D2-40, and podoplanin [61–63]. However, it should be kept in mind that LMs can be associated with the other vascular malformations.
LMs are present at birth in half of the patients (prenatal diagnosis of macrocystic types is possible) and are diagnosed before the age of 2 years in 90 % of the cases [61, 64]. They mainly involve the head and neck (55–95 %) and axillary regions (20 %) with a predilection for the left side of the body [64]. They can affect multiple structures (lung, intestine, liver, spleen, etc.) including the bones [38]. Macrocystic LMs are typically large and clinically manifest as a soft swelling mass with a rubbery consistency. They are well limited and mobile under a normal skin [57, 64]. Transillumination confirms the liquid nature of the lesion. The majority of the cases are asymptomatic, but complications, such as local compression, sudden increase in size secondary to bleeding (trauma), or infection from an adjacent process of the head and neck, can occur [65].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree