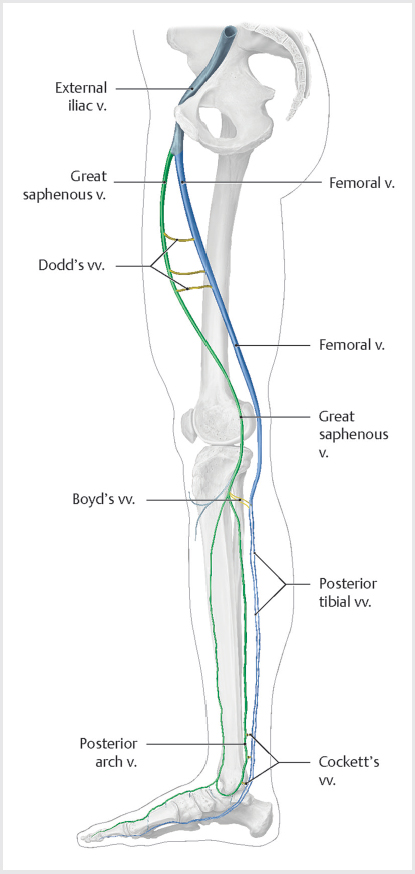
9 Venous Disease The lower extremity venous circulation has two major systems: superficial and deep. The superficial veins drain the skin and direct the flow of venous blood into the deep venous system via the perforator veins. While the superficial and deep veins run longitudinally, the perforaters run horizontally (▸Fig. 9.1, ▸Fig. 9.2). Problems with flow in the perforators can sometimes propagate into the superficial veins. The deep veins carry blood back toward the heart. In situations where the deep venous system is occluded, the superficial venous system can serve as a bypass circuit for venous return, albeit much less efficiently. Fig. 9.1 The superficial veins of the lower extremity drain blood from the skin and superficial tissues. Venous valves prevent blood from pooling and maintain normal lower extremity hydrostatic pressure. Patients with superficial venous disease most commonly have reflux in the greater saphenous vein, but the lesser saphenous veins (including the anterior and posterior accessory saphenous in the thigh and the small saphenous in the calf) may also be involved. (Source: 1.4 Superficial Veins. In: Collares F, Faintuch S, eds. Varicose Veins: Practical Guides in Interventional Radiology. 1st Edition. Thieme; 2017. doi:10.1055/b-006-160939) Fig. 9.2 Major perforators of lower extremity carry blood from the superficial veins into the deep veins. Perforators run horizontally, while the veins of the superficial and deep system run vertically. (Source: 1.5 Perforating Veins. In: Collares F, Faintuch S, eds. Varicose Veins: Practical Guides in Interventional Radiology. 1st Edition. Thieme; 2017.) A unique feature of medium-sized veins found in the extremities is the presence of valves. Along with the normal muscular contractions of leg muscles, these one-way flaps permit blood flow only toward the heart, preventing venous blood from pooling and exerting excessive hydrostatic pressure in the extremities. Venous thromboembolic disease (VTE) is a term that encompasses both deep venous thrombosis (DVT), as well as pulmonary embolism (PE). It is the third leading cause of cardiovascular death behind heart attack and stroke, and the leading cause of preventable hospital-related death. VTE can be a complex entity, owing to a wide range of patient presentations and severities (▸Table 9.1). As you read this section, keep in mind that the key to understanding VTE management is being able to differentiate simple from complex DVT, as much of the treatment strategy hinges on this determination. The symptoms most commonly associated with simple DVT are nonspecific and include extremity swelling, pain, and warmth. A swollen extremity may be caused by venous disease, lymphatic disruption, volume overload, and a number of other nonvenous conditions. Patients may have unilateral or bilateral symptoms, and in some cases may have no symptoms at all. The examination can be variable as well. Complicating matters further, patients may be evaluated in widely different clinical settings, making it difficult to learn a single approach to diagnosis. For example, in the inpatient setting, sometimes a search for DVT will be prompted by an unexplained leukocytosis—not the type of thing you would be thinking about for a patient seen in a primary care clinic. Given the occasionally ambiguous presentation, the patient’s signs and symptoms need to be interpreted in the context of DVT risk factors to raise clinical suspicion (▸Table 9.2). Virchow’s triad (venous stasis, endothelial injury, and hypercoagulable state) guides clinicians to the most common risk factors for VTE, and more exhaustive lists of risk factors generally implicate one of these three factors. If you’ve spent any time in the ED, you probably have familiarity with some objective tools used to determine the probability of DVT. Measuring a D-dimer can be helpful as a screening test but needs to be used appropriately. When you order a D-dimer, you should have an understanding how the result will guide your next step. Having a D-dimer come back in the normal range (“negative”) should give you confidence in ruling out DVT. Unfortunately, postsurgical and cancer patients (in whom DVT risk tends to be greatest) often have a high level of systemic inflammation and will almost always have an elevated D-dimer. In these cases, an elevated D-dimer is less useful. Table 9.1 Spectrum of VTE presentations
9.1 Venous Thromboembolic Disease
Work-up of Suspected Deep Vein Thrombosis
| DVT | PE |
Acute symptoms | Hypoxemia, tachypnea | Lower extremity pain, swelling, edema |
Acute complications | Phlegmasia, pulmonary embolism | RV dysfunction and obstructive shock |
Chronic complications | Post-thrombotic syndrome | Pulmonary hypertension (CTEPH) |
Abbreviations: CTEPH, chronic thromboembolic pulmonary hypertension; DVT, deep venous thrombosis; PE, pulmonary embolism; RV, right ventricular; VTE, venous thromboembolic. | ||
Prior DVT |
Malignancy |
Recent surgery |
Pregnancy |
Oral contraceptives |
Immobilization (travel history, trauma, etc.) |
Inherited coagulopathy |
Smoking |
Abbreviation: DVT, deep venous thrombosis. |
In reality, most clinicians rely on a clinical gestalt rather than a Wells score to determine when to image for suspected DVT. While not the most evidence-based approach, this is probably okay for a couple of reasons. One, an ultrasound is a relatively cheap test that can give a near definitive answer without any radiation exposure to the patient. Two, a missed diagnosis has the potential for serious complications, as will be discussed later.
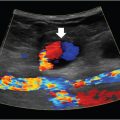
Compression ultrasound is the initial imaging modality of choice. Noncompressible veins are highly suggestive for the presence of DVT. The exam will also determine the location and extent of clot burden (important for management) and can differentiate between acute and chronic DVT. Acute DVTs tend to be anechoic within larger-caliber veins, and typically do not have identifiable collaterals. Chronic DVTs are more often echogenic, within a narrow vein, and are accompanied by collaterals. Elastography is being investigated as a novel method for determining clot chronicity, but it has not yet been validated.
The sensitivity and specificity of compression ultrasound for detection of DVT is high, and nondiagnostic studies are the minority. A nondiagnostic study can be repeated a few days later to clarify the findings. Rarely, CT or MR venography is used in cases of suspected DVT involving the iliac vessels or IVC (where ultrasound compression is not possible), in some cases where the DVT is considered complicated, and occasionally for nondiagnostic ultrasounds.
Once a DVT is confirmed by ultrasound, clot burden, location, and chronicity are used to determine the treatment strategy. With simple DVTs this is relatively straightforward. Unfortunately, not all DVTs are diagnosed before complications arise. The complications that result from untreated DVT can be categorized as local or embolic.
Local Sequelae of Deep Vein Thromboses
Local complications of DVTs can develop acutely as phlegmasia, or chronically as post thrombotic syndrome (PTS).
Phlegmasia results from massive deep venous occlusion, resulting in venous congestion, cyanosis, and critical limb ischemia of the extremity. Although it represents a small minority of cases, recognition is important as phlegmasia is considered a surgical emergency. If not treated appropriately, it can quickly progress to venous gangrene and limb loss. There is a higher incidence of phlegmasia in cancer patients who develop DVT, reflecting a profoundly hypercoagulable state and higher risk of extensive thrombosis.
Phlegmasia is recognized by the presence of unique physical exam findings. Phlegmasia alba dolens is characterized by a pale, white-appearing limb as a result of transient arterial spasm induced by the massive venous thrombotic event. Pulses may be absent, but limb loss is unlikely at this stage. About half of the patients with phlegmasia alba dolens progress to phlegmasia cerulea dolens, the more serious counterpart. This results when the thrombosis involves both the deep and superficial venous systems. With collateral flow provided by the superficial veins compromised, venous hypertension becomes extreme. The appearance of the extremity progresses from pale to cyanotic (▸Fig. 9.3). Venous hypertension and edema restricts arterial blood flow, leading to ischemia. Without prompt treatment, this almost invariably leads to venous gangrene, potentially requiring amputation.
Fig. 9.3 This patient presented with a swollen, cyanotic foot, consistent with a diagnosis of phlegmasia cerulea dolens. (Source: Case 135. A 66-year-old woman with diabetes underwent a coronary bypass 11 days prior. In: Ferral H, Lorenz J, eds. RadCases: Interventional Radiology. 1st Edition. Thieme; 2010.)
Phlegmasia is a rare complication of DVT. Far more common is PTS, which can develop months after an acute DVT. Proximal DVTs, particularly ileofemoral, have the highest risk of causing PTS (nearly half the patients end up with PTS in 1 to 2 years). This condition is characterized by a constellation of symptoms, including pain, swelling, heaviness, fatigue, skin pigmentation, and possibly skin ulceration when severe. PTS is caused by damaged venous valves, with resulting reflux and pooling of blood in the distal veins, and ultimately venous hypertension. In mild cases, symptoms of PTS are similar to those of acute DVT, so evaluation for PTS should not be performed for at least 3 months after an acute DVT.
The diagnosis of PTS is clinical, usually apparent when classic symptoms develop several months after a documented DVT. The Villalta scale is a tool used to grade the severity, and can be found online for reference. Patients with a score less than 5 indicates no PTS, 5 to 14 is consistent with mild-to-moderate PTS, and 15 or more (or the presence of a venous ulcer) defines severe PTS.
The quality of life in these patients is poor, and unfortunately there is no cure for PTS. Symptom management typically involves compression stockings, leg elevation, and exercise. In experienced centers, endovascular techniques can be used to improve (but not cure) PTS symptoms. For this reason, PTS prevention is an important consideration in every patient diagnosed with a DVT.
Pulmonary Embolism
Though pulmonary embolism can result from other causes, the vast majority of PEs are a complication of DVT. Despite the association between these two conditions, PE can sometimes present in the absence of symptomatic DVT.
The pathophysiology of PE and development of symptoms involves both the circulatory and respiratory systems. When thrombus in the pulmonary artery is large enough, pulmonary artery pressure increases, resulting in increased right ventricle size and decreased cardiac output. The response to decreased cardiac output is a sympathetic nervous system mediated, compensatory increase in heart rate and systemic vasoconstriction. The thinner-walled right ventricle is not suited for pumping against high pressures in the pulmonary circulation, and right-sided heart failure can result if the pulmonary hypertension is severe enough. If the obstruction does not improve, progressive right ventricular fatigue will eventually lead to right-sided heart failure, compromised cardiac output, and systemic hypotension.
From a respiratory standpoint, the presence of thrombus results in V/Q mismatch. The parts of the lungs unaffected by the embolus receive a compensatory increase in blood flow, so much that normal ventilation can’t keep up and hypoxemia develops. Platelet-derived factors released due to the presence of clot can cause edema of the healthy lung, which further worsens oxygenation. There are multiple ways in which hypoxemia develops in these patients, so it makes sense that dyspnea is the most common symptom in PE patients. Hemoptysis and chest pain are not as common, but can be seen when the PE results in pulmonary infarct.
The severity of PE can vary dramatically depending on the clot burden, as well as the chronicity and the patient’s baseline cardiopulmonary health. Some may be completely asymptomatic, while others may die within minutes of an embolic event. As with DVT, pulmonary embolism produces a varied clinical picture for different patients. It’s also investigated in very different settings and with different levels of suspicion. This can make it difficult to adopt one single approach to diagnosis. We’ll focus on a patient presenting to the emergency room.
The differential for patients coming to the ED with dyspnea and chest pain is broad. All patients will get a basic work-up before any targeted diagnostics for PE. A chest X-ray in a patient with PE can be completely clear or may show nonspecific findings such as atelectasis. Only in a minority of cases it will show a relatively specific finding for PE, like a Hampton’s hump (indicating pulmonary infarct). An ECG may demonstrate an S1 Q3 T3 morphology, and arterial blood gas may reveal a hypocapnic, hypoxemic respiratory alkalosis. Bear in mind that even large pulmonary emboli can be present without any of these findings.
Making a clinical diagnosis of PE is difficult in all but the most obvious cases, so it usually takes a combination of risk factors and lack of better alternative diagnoses to narrow down those patients who need further work-up. Analogous to that for DVTs, there is a Wells score available for estimating the pretest probability of PE. If there is low probability, a D-dimer can be used to rule it out. If there is a high pretest probability based on the Wells criteria (score > 4), a CT pulmonary angiogram (aka PE study) is the next step. Alternatively, some clinicians use the PERC rule. The PERC rule is a validated set of criteria that, if met, indicate the risk of PE is sufficiently low to forgo imaging.
When used in conjunction with the Well’s score for patient selection, a technically adequate CT pulmonary angiography (CTPA) is both sensitive and specific for the detection of PE, carrying a negative predictive value that approaches 100%. When the study is negative, it can often be valuable in determining an alternative cause for the patient’s symptoms. When positive, it can aid in determining the severity of PE by quantifying the amount of clot and identifying any evidence of pulmonary infarct or right heart strain (▸Fig. 9.4). Perhaps because of how useful the study has proven to be, the number of CTPAs has increased dramatically in the past two decades (including in patients who fall in the low pretest probability group).
Unlike the ultrasound for DVT diagnosis, CT exposes the patient to radiation and the risks associated with contrast administration. Additionally, liberal use of CTPA in the low-probability setting greatly increases the false-positive rate, which leads to inappropriate and avoidable treatment. This is something to keep in mind as you might find yourself in an IR practice that admits to its own service, and faced with a questionable PE in a postoperative patient.
For those patients who cannot get CTPA, due to severe contrast allergy or any other reason, a V/Q scan is another option. Pregnant patients with suspected PE get a V/Q over CTPA only when the chest X-ray is normal. A V/Q scan may be interpreted as low probability, intermediate probability, or high probability. A low-probability scan in a patient with low pretest probability needs no further work-up. A high-probability scan in a high pretest probability patient is considered positive for PE. Any other combination of discordance between the V/Q scan and the pretest probability is considered indeterminate. These patients can be further evaluated with compression ultrasound of the extremities. If DVT is identified, empiric treatment of PE is appropriate. When there is discrepancy between the ultrasound finding and the clinical suspicion for PE, these patients are evaluated on a case-by-case basis in light of the risks and benefits of treatment.
PEs are categorized as massive, submassive, or low risk. In massive PE, the patient has evidence of right heart strain and is hypotensive, often requiring inotropic support. In submassive PE, the patient has right heart strain, but is normotensive and hemodynamically stable. Low-risk patients have neither right heart strain nor hypotension, but may be symptomatic in other ways.
Right heart strain can be determined in a number of different ways. Suggestive findings on the CTPA include flattening of the interventricular septum, right ventricular (RV) enlargement, and reflux of contrast (▸Fig. 9.5). Bedside ultrasound will show similar findings. Elevated biomarkers, including troponin and pro–B-type natriuretic peptide (pro-BNP), are also suggestive, especially when there are simultaneous imaging clues.
In most adequately treated PEs, pulmonary vascular physiology and right heart strain will return to normal after the pulmonary artery thrombus is resorbed by the body. In a small minority of patients, the pulmonary artery thrombus persists, organizes, and leads to a state of pulmonary hypertension. This condition is known as chronic thromboembolic pulmonary hypertension (CTEPH).
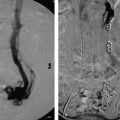
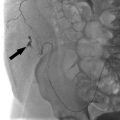
Fig. 9.4 This postoperative patient developed sudden-onset dyspnea and tachycardia overnight. CT pulmonary angiography study was obtained which showed a right-sided subsegmental pulmonary embolism (arrow).
Fig. 9.5 A patient with a history of pancreatic cancer developed sudden-onset dyspnea and chest pain. After ruling out a myocardial infarct, a CT pulmonary angiography study was obtained which showed a large pulmonary embolism (PE) in the left main pulmonary artery (arrow). A subsequent echocardiogram identified findings of right heart strain, not unexpected for the size of this PE.
Management of Venous Thromboembolism
The goals of DVT management include (1) symptom relief, (2) acute PE prevention, (3) recurrent DVT prevention, and (4) PTS prevention. When PE is the presenting problem, the treatment strategy includes the above, acknowledging the likelihood of concurrent DVT, but with a more pressing goal of preserving or restoring the patient’s hemodynamic status.
Oral anticoagulation is the most common treatment for both DVTs and PEs. Anticoagulation does not directly break down clot, but does prevent propagation of the thrombus while the body naturally lyses it. For patients who need PE prophylaxis but cannot be anticoagulated or in whom anticoagulation has failed, an IVC filter may be an alternative. In cases of complicated VTE, certain patients benefit from venous recanalization with either endovascular therapy (catheter-directed therapy) or systemic thrombolytics.
The options available for anticoagulation include the following:
1. Parenteral agents such as unfractionated heparin or argatroban (for heparin allergy or history of heparin-induced thrombocytopenia).
2. Subcutaneous agents such as low-molecular-weight heparin (LMWH; enoxaparin) or fondaparinux.
3. Oral anticoagulants including warfarin (vitamin K antagonist) and direct oral anticoagulants (DOACs) such as rivaroxaban/apixaban (factor Xa inhibitors) or dabigatran (direct thrombin inhibitors).
In 2016, the American College of Chest Physicians (ACCP) released updated guidelines on antithrombotic therapy and anticoagulant selection. According to those recommendations, cancer patients requiring anticoagulation for VTE should be put on LMWH. Noncancer patients should preferentially be put on a DOAC over warfarin.
DOACs come with certain advantages over warfarin, including less drug interactions. Warfarin’s drug interactions can lead to a supratherapeutic international normalized ratio (INR) and are a huge headache for clinicians. Further, DOACs do not require routine monitoring as warfarin does. This is great for patients, but it comes with the price of not being able to determine patient compliance.
A few other disadvantages of DOACs should be noted. The majority of the DOACs are renally cleared, and should be used with caution in patients with renal impairment. They are contraindicated in patients with severe renal impairment. Most clinicians will anticoagulate patients with end-stage renal disease (ESRD) with unfractionated heparin and warfarin (not LMWH, since it is also renally cleared). Finally, some of the DOACs do not have reversal agents, though these are expected to be available in the near future. Already, dabigatran has a reversal agent (idarucizumab). For the other DOACs (apixaban, rivaroxaban, edoxaban), prothrombin complex concentrate can be used if immediate reversal of anticoagulation is required. The half-life of the DOACs is relatively short (~ 12 hours), and generally does not require reversal in most scenarios.
Management of Deep Vein Thromboses
Not all DVTs need to be treated (▸Fig. 9.6). Distal DVTs (below the knee in the anterior/posterior tibialis, peroneal, and deep muscular veins) generally have a lower risk of embolization, and treatment is usually not necessary. There are a few key exceptions. Symptomatic distal DVTs and patients with risk factors for proximal extension should be treated. Risk factors for proximal extension include DVT in more than two veins, thrombus just distal to the popliteal vein, thrombus that is notably large in either diameter or length, patients with active cancer, inpatients, and those with unprovoked DVT.
Asymptomatic patients and those without risk factors for extension should get follow-up with serial ultrasound exams until resolution is ensured. Proximal DVTs (above the knee in the iliac, femoral, or popliteal veins) confer a higher risk of complications and should all be treated.
Simple DVTs are treated in the outpatient setting. The most common scenario when a DVT is diagnosed in the ED is for the patient to be put on LMWH until warfarin becomes therapeutic, or just simply started on a DOAC. Patients on LMWH/warfarin follow-up with their primary care physician or a specialized warfarin clinic for INR checks every couple days during this transition. DOACs have rapid onset to therapeutic activity and therefore do not need to be bridged with LMWH. For this reason, DOACs are becoming increasingly popular for treatment of simple DVTs.