and John P. Deveikis2
(1)
Department of Surgery, Division of Neurosurgery, and Departments of Radiology and Neurology, University of Alabama, Birmingham, AL, USA
(2)
Bayfront Medical Center, St. Petersburg, FL, USA
Abstract
This chapter covers the gamut of intracranial venous issues, ranging from the most common entity – developmental venous anomalies – to the nonexistent: “chronic cerebrospinal venous insufficiency.”
This chapter covers the gamut of intracranial venous issues, ranging from the most common entity – developmental venous anomalies – to the nonexistent: “chronic cerebrospinal venous insufficiency.”
16.1 Developmental Venous Anomalies
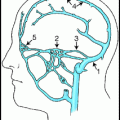
Developmental venous anomalies (DVA) (aka venous angiomas, venous malformations, venous anomalies, medullary venous malformations, or caput medusae) are a normal variant of intraparenchymal medullary veins, being larger and more visible on imaging than most medullary veins (Fig. 16.1).1 They are usually found in white matter and are associated with cavernous malformations.2 They are characterized histologically by a complex of sometimes thickened and hyalinized veins with interspersed normal neural parenchyma.3
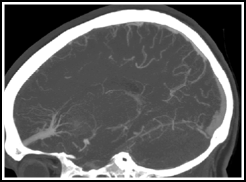

Fig. 16.1
Frontal lobe venous angioma in a sagittal CT angiogram reconstruction. It has the typical stellate network of veins converging on a single collecting vein.
The most important thing to know about DVAs is that they are usually incidental findings on imaging, and with rare exception, are completely benign.4–6 When associated with a cavernous malformation, they may be erroneously identified as the source of symptoms.7 They may also be confused with AVMs and other aggressive lesions by the uninitiated. The authors of this handbook wish they had a dollar for every patient referred to them with an incidental DVA found on imaging and misidentified as an AVM.
16.1.1 Epidemiology
16.1.2 Aetiology
16.1.3 Imaging
1
Typical appearance on MRI and CTA: radial pattern, the so-called caput medusa, which converges on an enlarged central trunk.14
2
Seen only during the venous phase on angiography.
3
About two thirds are in a supratentorial location and one third are in the cerebellum and brainstem.11
4
Often found in close proximity to a cavernous malformation.
5
Of all patients found to have a venous angioma on MRI, 18% had an associated cavernous malformation.11
16.1.4 Natural History and Presentation
1
A prospective study with 298 patient-years of observation found a symptomatic haemorrhage rate of 0.34% per year.15
2
In every case in which there was a haemorrhage associated with the venous angioma, a cavernoma was present.11
3
Rarely, spontaneous thrombosis of a very large venous angioma may cause extensive venous infarction or haemorrhage.16
16.1.5 Arterialized Venous Malformations
A rare condition associated with DVAs is the so-called arterialized venous malformation (aka venous-predominant AVM, or atypical DVA).17 These consist of a typical appearing DVA that is associated with arteriovenous shunting. On angiography they show no AVM nidus, but have a homogeneous arterial blush with early appearance of the medullary veins in the DVA.17–19 (Fig. 16.2) On MR, the typical spoke-wheel appearance of a DVA is seen.20 These lesions are quite rare, making it difficult to characterize the natural history. The largest series17 includes 15 patients: 6 lesions were asymptomatic, 8 caused haemorrhage, and 1 caused seizures without haemorrhage. After surgical excision, there were 2 post-op haemorrhages, highlighting the inherent danger in trying to remove a DVA.
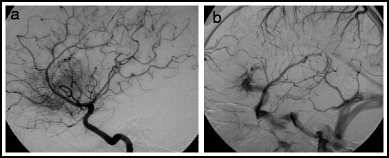

Fig. 16.2
Angio-pathologic correlation! Arterialized developmental venous anomaly. Lateral right internal carotid arteriogram. Early arterial phase (a) shows prominent blush in the frontal region, but no shunting. Venous phase (b) shows typical DVA draining that area.
16.1.6 Management
1.
Most DVAs are incidental findings and do not require treatment or follow-up imaging.
2.
During surgery for evacuation of a haemorrhage or resection of a cavernous malformation, it is critical to preserve the associated venous angioma, particularly in the brainstem.
3.
Removal of a venous angioma may cause a venous infarction.
16.1.6.1 Global Gem
Pierre Lasjaunias (1948–2008) was a pioneering Parisian neuroradiologist and neurointerventionalist who was notable for exploring the venous side of cerebrovascular development and disorders, among many other accomplishments. He was probably most famous, along with Alex Berenstein and Karel ter Brugge, for the five-volume epic text Surgical Neuroangiography, and for his work in the understanding and management of vein of Galen malformations. Discussion of food, wine, and cerebrovascular disease is much more impressive with a French accent.
16.1.6.2 Venous Anomalies in Vein of Galen Malformations
1.
Vein of Galen Malformations are an arteriovenous shunt into the wall of a dilated vein of Galen. (see Chap. 14)
2.
This “vein of Galen” may actually be a fetal median prosencephalic vein of Markowski, which is normally present between 7 and 12 weeks gestation.1 This is a subarachnoid venous structure that usually drains not into the straight sinus, but into a more cranially directed falcine sinus which then empties into the superior sagittal sinus.
4.
Due to the high flow shunts associated with the fistula, on angiography, deep veins (e.g. internal cerebral veins and basal vein of Rosenthal) are usually not seen draining into the vein of Galen in this condition. This was used historically as a justification for doing transvenous embolization of the venous pouch in vein of Galen malformations.
5.
Au contraire, mon ami. There are increasing reports indicating visualization of normal deep veins draining into the prosencephalic vein after curative transarterial embolization of the fistula.5
6.
This deep venous drainage that may not be visible angiographically may explain the relatively poor clinical outcomes achieved with transvenous embolization or open surgical treatment of vein of Galen malformations.6
16.2 Sinus Pericranii
Sinus pericranii is a rare venous condition that consists of a large communication between an intracranial venous sinus and an anomalous extracranial venous channel.21 The extracranial component is a soft, fluctuant scalp mass that is compressible and enlarges in a dependent position or with Valsalva maneuver.21,22 A young boy with this finding was described by Percival Potts in 1760.22 The condition was called sinus pericranii by Stromeyer in 1850.23 Other terms applied to the lesion included fistula osteovasculare and varix sprius circumscriptus venae diploica frontalis. 24
16.2.1 Epidemiology
16.2.2 Aetiology
1.
Congenital
b.
2.
3.
Other acquired causes
e.
A special category is sinus pericranii associated with craniosynostosis. These lesions can spontaneously resolve after surgical repair of the craniosynostosis. The term, “squeezed-out sinus syndrome” is used in this case.41
16.2.3 Natural History and Presentation
1.
Most present as an unexplained painless, soft, fluctuant swelling on the head, possibly with associated blue discoloration of the overlying skin.
4.
16.2.4 Imaging
1.
CT without contrast can show the slightly hyperdense venous malformation and bone windowing can reveal the bony defect through which the communication to the sinus passes.22
2.
3.
The lesions can be seen during the venous phase of cerebral angiography, although oblique angles may be necessary to best demonstrate the connection to the intracranial sinus.43
4.
3D angiography is particularly helpful to allow optimal visualization of the transcranial connection and the associated bony opening.26
5.
Percutaneous puncture of the subgaleal venous channel with contrast injection can also show the connection to the venous sinuses.26
16.2.5 Management
1.
Some advocate aggressive management due to a risk of life-threatening complications after trauma, such as bleeding, infection and air embolism.43,47
a.
Patients with sinus pericranii should be counseled to avoid being stabbed, shot, or bludgeoned to avoid those complications.
b.
In reality, most lesions are treated for purely cosmetic reasons.
2.
3.
Percutaneous sclerotherapy of the extracranial venous anomaly may be done for cosmetic reasons, but this is not often curative and transient sixth nerve palsy with this technique has been reported.44
4.
5.
Caveat: When angiography shows that there dominant flow of the intracranial veins preferentially via the sinus pericranii, it is best to treat the lesion conservatively.21
16.3 Venous Malformations
Venous malformations are low-flow congenital vascular anomalies that frequently involve the skin, subcutaneous fat and/or muscle. Especially in the head and neck, they can produce such profound deformity that they can cause serious psychological and social problems for the patient.49 In addition to cosmesis, they can also have functional implications in the orbit and around the airway. They have often been indiscriminately grouped together with various vascular lesions under the term “vascular birth marks,” and are frequently misdiagnosed as haemangiomas (Table 16.1).
Table 16.1
Superficial venous malformations vs. capillary (infantile) haemangiomasa
Venous malformations | Capillary haemangiomas | |
|---|---|---|
Definition319 | Low-flow vascular malformation. | Benign vascular neoplasm. |
Symptom onset319 | At birth | Soon after birth |
Growth pattern54 | Slow growth throughout life. May have growth spurts at puberty, pregnancy | Rapid proliferative phase in early infancy, followed by phase of involution after 12 months of age. |
Margins54 | Poorly defined | Usually well defined |
Flat endothelium forming dysplastic vessels. Does not proliferate in vitro. | Densely packed endothelial cells in clusters. Proliferates in vitro. | |
Treatment54 | Surgery, sclerotherapy, conservative therapy. | Steroids, propranolol, surgery, conservative therapy. |
16.3.1 Epidemiology
5.
Location: Often seen in the head and neck, including the tongue, the parotid region, the orbit, as well as virtually anywhere in the body.54
16.3.2 Pathophysiology
1.
These are congenital anomalies that are present at birth.
2.
3.
The anomalous vessels dilate, causing local swelling. These can partially thrombose, causing venous outlet obstruction and dilating the remaining vascular spaces even further.
4.
Perivascular lymphocytic infiltrates suggests inflammation, also a mechanism contributing to symptomatic swelling and pain.56
16.3.3 Syndromes Associated with Venous Malformations
1.
Klippel-Trenaunay syndrome.
a.
b.
Although case reports have suggested an association between Klippel-Trenaunay syndrome and spinal arteriovenous malformations, this association does not appear to be valid.59
c.
There has been a case combining features of KTS, Sturge Weber, and phakomatosis pigmantovascularis, suggesting that these entities may be part of a spectrum of disease.60
2.
3.
Blue rubber bleb naevus syndrome
a.
A rare inherited condition with multiple raised cutaneous and gastro-intestinal tract venous lesions.35
16.3.4 Natural History and Presentation
1.
Venous malformations are frequently noted at birth and appear as an area of soft tissue swelling. Often with associated bluish discoloration of the skin and sometime with visible abnormal venous spaces.
a.
The lesion is non-pulsitile. A thrill or bruit would suggest an arteriovenous malformation.
b.
Swelling worsens when the lesion is in a dependent position, during Valsalva maneuver, or during crying.
2.
3.
The lesions tend to involve muscles and can cause pain and limitation of movement related to the muscle.
5.
Lesions near the mouth or pharynx can cause difficulty swallowing.
6.
Those near the airway can cause dangerous difficulty breathing.
7.
Lingual and other oral lesions can cause bleeding in children when they suckle.
16.3.5 Imaging
1.
The appearance of venous malformations on CT without contrast can be indistinct and confusing. Calcification is common. Contrast-enhanced studies confirm the vascular nature of the lesion.56
2.
4.
Catheter angiography is not needed, since, at best, it may only show irregular venous structures very late in the venous phase.
5.
Direct needle puncture of the lesion with contrast injection shows very abnormal vascular spaces.73
16.3.6 Management
1.
2.
Conservative therapy
a.
Analgesics and anti-inflammatory medications as well as selective use of compressive dressings or clothing can ameliorate some symptoms of venous malformations.
b.
Lesions that are small and minimally symptomatic can easily be treated conservatively.
c.
Many of these measures can be used to complement more invasive therapy.
3.
Sclerotherapy
a.
Technique: percutaneous puncture of the abnormal venous channels and injection of a sclerosing agent:
d.
Complications may be lowered by placing a second needle in the vascular space to decompress it as the ethanol is injected.77
f.
Series comparing ethanol to bleomycin as the sclerosing agent show slightly less efficacy with bleomycin but considerably lower complication rates.79
4.
Neodynium-YAG laser therapy
16.4 Venous Stenosis
Recognition of venous stenosis and its association with various disease processes is an emerging field with only a few isolated cases reports or very small series, and limited understanding of the significance of venous disease.
16.4.1 Idiopathic Intracranial Hypertension
Idiopathic intracranial hypertension (IIH) (a.k.a psuedotumour cerebri, benign intracranial hypertension) is a potentially disabling condition producing symptoms of increased intracranial pressure, with absence of an underlying mass lesion to explain the symptoms.
The Dandy criteria for diagnosis include83
1.
CSF pressure >25 cm H2O
2.
No localizing neurological signs (except abducens palsy)
3.
Normal CSF composition, and
4.
Normal to small ventricles on pneumoencephalography.
Since the CT and MRI imaging became available, amendments to the Dandy criteria include imaging to exclude obstructive hydrocephalus,84 and venous thrombosis as causes of the increased intracranial pressure.85 Small series of patients with signs of IIH and imaging evidence of dural venous sinus stenosis has prompted some to propose a causal relationship between venous stenosis and IIH.86
16.4.2 Statistics
1.
2.
3.
16.4.3 Aetiology
Idiopathic intracranial hypertension by definition has no known cause, but there are several theories. In fact, whenever a convincing aetiology for the symptom complex is finally found, the term idiopathic intracranial hypertension no longer applies.
1.
Cerebrospinal fluid malabsorption
c.
This theory has prompted the development of and is supported by positive outcomes from CSF diversion techniques including repeated therapeutic lumbar punctures and ventriculo-peritoneal shunting.
d.
Skeptics argue that there is no evidence of ventricular dilatation in IIH, whereas other conditions manifesting abnormal CSF production or absorption usually are associated with imaging evidence of hydrocephalus.95
2.
Cerebral blood flow dysregulation
a.
b.
One wonders why acetazolamide would help in these patients since that drug increases cerebral blood flow.
c.
Decreased cerebral blood flow seen in some patients correlates with the presence of venous outflow obstruction, and the disease in this subgroup of patients has been called secondary intracranial hypertension (SIH).97
3.
Causes of venous outflow obstruction
a.
Increased intra-abdominal pressure
i.
Animal models show a direct correlation between intracranial pressure and intra-abdominal pressure.99
ii.
Increased abdominal pressure in obese IIH patients may be transmitted to the intracranial venous system.
iii.
Small series show transient symptom relief in obese IIH patients when an external device was used to lower abdominal pressure.100
iv.
Skeptics say this theory does not explain the female predominance in IIH and that hormones may have a role as well.101
b.
Venous sinus stenosis
i.
Given the role of dural venous sinuses in the removal of cerebrospinal fluid from the intracranial compartment, and the observation of elevated venous pressures on intracranial venography in 10 out of 10 patients with pseudotumour, Karahalios and coworkers proposed that venous hypertension may be the final common pathway for production of elevated CSF pressure.86
ii.
An MRV study showed significant bilateral venous sinus stenoses in 27 of 29 IIH patients compared to 4 of 59 matched control subjects.102
iii.
iv.
An interesting study showed coupling of CSF and superior sagittal sinus pressure, suggesting the potential for a vicious cycle, in which high CSF pressure raises venous pressure, which creates a further worsening of CSF pressure.107
v.
On the other hand, in a study of nine patients with IIH and venous sinus stenosis, the stenosis persisted even after normalization of CSF pressure, medically and by lumbar puncture.108
16.4.4 Natural History and Presentation
16.4.5 Imaging
By definition, idiopathic intracranial hypertension is a diagnosis of exclusion, and imaging should be done to exclude other causes of increased intracranial pressure such as tumors, vascular malformations, inflammatory processes, and venous sinus thrombosis.
16.4.6 Management
a.
Traditional therapy includes medical therapy with analgesics, corticosteroids, acetazolamide, cerebrospinal fluid diversion with periodic lumbar puncture or ventriculoperitoneal shunting, and optic nerve sheath decompression to relieve pressure on the nerve.114
b.
Weight loss can be helpful
c.
d.
Although there are no prospective randomized trials of surgical intervention, a systematic review found improved or resolved visual deficits in 38% of patients after VP shunting, 44% of patients after lumboperitioneal shunting, 47% of patients after venous stenting, and 80% of eyes after optic nerve fenestration.124
e.
More recently, a series of 52 patients stented showed a 100% success rate of improving signs and symptoms of papilloedema and headache.125 There were six recurrences requiring additional stent placement, but no complications.
16.4.7 Venous Tinnitus
1.
Continuous “whooshing” sound accentuated in systole and is heard by the patient and may even be audible to the examiner.126
2.
Venous tinnitus frequently appears as neck bruit, as opposed to a venous hum, which can be heard in half of normal individuals and even more commonly in pregnant women.127
3.
4.
Features supporting a venous aetiology in patients with tinnitus include increased prominence of the sound when the patient is recumbent and compressing the contralateral jugular vein, as well as dampening of the sound by compressing the ipsilateral jugular.
5.
Causes:
b.
c.
d.
Some cases are idiopathic
6.
Treatment
a.
Tinnitus related to systemic disease usually improves after treatment of the underlying condition.
b.
Similarly, resolution of the increased intracranial pressure can improve the tinnitus.
c.
d.
Stenting of transverse sinus stenosis can treat the tinnitus and does not impair venous outflow.116
e.
Stenting is preferable to jugular ligation and should be considered in patients with classic venous tinnitus ipsilateral to a significant stenosis confirmed on direct retrograde venography (see Chap. 12).
16.4.8 Venous Stenosis and Arteriovenous Fistulas and Malformations
Venous structures play an important role in cerebral arteriovenous fistulas and arteriovenous malformations (see Chap. 15). There is increasing recognition of the role of venous structures in the development these lesions and their evolution over time.
1.
Venous occlusive disease is a factor in development of arteriovenous fistulas.
a.
b.
Venous hypertension appears to be the main factor require for fistula development, since thrombosis without venous hypertension is not sufficient.145
c.
Genetic thrombophyllic abnormalities are seen more commonly in patients with dural arteriovenous fistulae than in the general public.147
d.
e.
A new dural-pial arteriovenous malformation developed 29 months after superior sagittal sinus and transverse sinus thrombosis.152
2.
Venous occlusive disease can contribute to increased risk of haemorrhage and/or worsening symptoms from intracranial AVMs.
a.
Venous occlusive disease can redirect flow in dural arteriovenous fistula toward cortical veins and that can affect symptomatology.153
b.
Computer modeling of brain AVMs shows impairment of venous drainage increases the risk of haemorrhage.154
c.
d.
Three out of four cases of transverse or sigmoid sinus dural AVF’s were completely cured by stenting after partial transarterial embolization, and some even without adjunctive embolization.157
3.
High-flow venopathy
16.4.9 Venous Stenosis and Its Relationship (or Lack Thereof) to Multiple Sclerosis
The notion that multiple sclerosis (MS) may be related to venous stenosis has received considerable attention since 2008. It is also very controversial.161 The concept of chronic cerebrospinal venous insufficiency (CCSVI) holds that insufficient intracranial venous outflow leads to a build-up of cerebral venous pressure and the changes associated with multiple sclerosis. The leading proponent of this theory is Paolo Zamboni, an Italian vascular surgeon.
1.
Chronic cerebrospinal venous insufficiency (CCSVI)
a.
Zamboni and colleagues162 proposed venous flow criteria for the diagnosis of CCSVI based on color Doppler criteria:
i.
Reflux in internal jugular and vertebral veins in sitting and supine position.
ii.
Reflux in deep cerebral veins.
iii.
Visible jugular vein stenosis on B-mode images
iv.
No flow in jugular or vertebral veins.
v.
If the cross-sectional area in the jugular becomes greater in upright than the supine position.
b.
Using these Doppler criteria, patients with multiple sclerosis in Zamboni’s studies showed significantly more of these criteria compared to controls, suggesting that the demylenating disease is causally related to venous abnormalities.
c.
Using susceptibility-weighted MRI imaging, a study of 16 multiple sclerosis patients showed greater iron deposition compared to 8 control patients, and this was interpreted to be evidence supporting CCSVI’s deleterious effects on the brain parenchyma.163
d.
The same group proceeded to an open label trial of venous angioplasty, with no control group.164 Of a total of 65 patients treated, the 35 relapsing remitting patients showed significant improvements in relapse free rates, fewer enhancing lesions on MRI and improved quality of life measures. Less improvement was seen in secondary progressive and primary progressive patients. Restenosis at the angioplasty site occurred in 47% of patients.
2.
The carnival of publicity concerning CCSVI
a.
A recent Google search for CCSVI reveals over one million possible sites. Many are posts to multiple sclerosis patient forums, informational sites (including www.ccsvi.org where the logo states “Opening veins, opening minds.”) and also doctors, hospitals and clinics advertising and offering the procedure.
b.
The popular media have reported on the topic. Many of the web sites and media reports use Zamboni’s term “liberation procedure” when referring to the venous angioplasty.
c.
This simple solution to a complex disease appeals to patients and especially those that are suspicious of the motives of the neurologists and multiple sclerosis researchers.165
d.
This procedure costs many thousands of dollars and is not covered by insurance in the United States.
3.
Evidence against CCSVI
a.
A larger series evaluating the prevalence of CCSVI characteristics among multiple sclerosis patients showed lower prevalence than earlier studies (56%) compared to 43% in other neurological disorders, prompting the authors to conclude that CCSVI does not have a causative role in MS.166
b.
c.
d.
The angioplasty procedures are not without risk. Jugular venous thrombosis has been reported following the “liberation procedure”.172
e.
The CCSVI criteria are based on Doppler ultrasound imaging, which is operator-dependent.
f.
Venous anatomy is extremely variable and extracranial veins are easily compressed and distorted.
g.
On a theoretical basis, Khan and coworkers173 took issue to the reasoning behind CCSVI according to the following arguments:
i.
MS is more common in young women, and chronic venous disease is not.
ii.
All the well-known epidemiological associations with MS including geography, ethnicity, vitamin D levels, etc. are not related to venous outflow insufficiency.
iii.
As shown elsewhere in this chapter, venous occlusive disease causes haemorrhagic infarction, edema, and increased intracranial pressure, which are not signs of MS.
iv.
Venous occlusive disease would be expected to progress over time, yet the natural course of MS trails off over time.
v.
Immune disorders elsewhere in the body do not have a venous basis.
vi.
Transient global amnesia has been associated with jugular venous reflux, but does not appear to be associated with MS.
vii.
A model that should confirm the association of MS with venous disease is the radical neck dissections performed for cancer treatment. The jugular veins are occluded and secondary occurrence of MS in this setting has not been observed.
4.
Recommendations
a.
Multiple sclerosis patients should undergo venous angioplasty procedures only in well-designed clinical trials.
16.5 Cerebral Venous Thrombosis
Cerebral venous thrombosis (CVT) can occur in the form of cortical venous thrombosis, venous sinus thrombosis, deep venous thrombosis, jugular venous thrombosis, or various combinations of the above.
16.5.1 Epidemiology
16.5.2 Aetiology
2.
Predisposing factors
16.5.3 Genetics
A comprehensive meta-analysis that evaluated 1,183 CVT cases and 5,189 controls in 26 studies revealed associations with genes involved in the clotting cascade:196
a.
Factor V Leiden/G1691A: Pooled odds ratio (OR) 2.40 (95% CI 1.75–3.3 p < 0.00001) CVT cases attributable to this polymorphism 6.8%.
b.
Prothrombin/G20210A: Pooled OR 5.48 (95% CI 3.88–7.74 p < 0.00001) CVT cases attributable: 14.2%.
c.
Metylene tetrafolate reductase/C677T: Pooled OR 1.83 (95% CI 0.88–3.80 p < 0.09) CVT cases attributable: 17.9%.
d.
Associated risk of CVT with these genes is greater in adults than children.
e.
A non-significant increased association with venous compared to arterial ischemia.
16.5.4 Imaging
1.
General concepts
a.
May see generalized brain edema or more focal edema that is not in a typical arterial territory. Territories involved:
i.
Sagittal sinus thrombosis: Parasagittal cortex bilaterally.
ii.
Transverse sinus thrombosis: Ipsilateral temporal and occipital lobes, and even the ipsilateral cerebellar hemisphere.
iii.
Deep cerebral venous thrombus: Bithalamic region oedema.
iv.
Cortical vein thrombosis: More localized cortical oedema.
b.
As the impairment of blood flow progresses to venous infarction, further oedema and haemorrhagic transformation may occur.197
2.
CT
a.
Non-contrast CT may show hyperdense clot in the thrombosed vein or sinus.
b.
CT angiography can be timed to optimize visualization of the venous sinuses (CT venography)
c.
CT venography is less prone to artifact compared with MR venography
3.
MR
a.
T2 and FLAIR sequences show oedema.
b.
T1 may show high signal clot in venous structures and bleeding in the brain
c.
Susceptibility-weighted imaging (gradient echo or T2*) is very sensitive in detecting haemorrhage.
4.
Angiographic findings:
a.
Sluggish arteriovenous transit and congested veins
b.
Absence of flow in thrombosed veins
c.
Filling defects may be seen in partially occluded venous structures but care should be taken to not misinterpret unopacified blood flowing in from arterial territories that were not injected.
d.
Direct catheter venography can confirm the presence of clot, but flow patterns can sometimes simulate solid filling defects (see Chap. 12)
16.5.5 Presentation and Clinical Features
1.
Protean manifestations include headaches, decreasing visual acuity, declining cognitive ability, hemiparesis, hemianaesthesia, aphasia, disturbed consciousness, coma, and even death.
3.
Delay in diagnosis is common: A study of 91 patients admitted for CVT showed the median time of hospital admission after symptom onset was 4 days, and only 25% were admitted within the first 24 h.200
4.
When there is a clinical suspicion for CVT, imaging confirms the diagnosis 16.3% of patients.201
5.
Mortality
c.
Patients over 65 years old had a 27% chance of death and 22% chance of dependency compared to 7% death and 2% incapacitation in younger adults.202
e.
On the other hand, a study of CVT in neonates showed 5 out of 90 died and a full 61% had a bad outcome.204
f.
Factors predicting a higher risk of death include:175
i.
Seizures, coma, disturbed consciousness, deep cerebral venous thrombosis, right-sided intraparenchymal bleed, posterior fossa involvement, and progressive focal neurological deficits.
g.
Transtentorial herniation is the most common immediate cause of mortality in the acute phase of the disease.205
16.5.6 Management
1)
Medical management
a)
Isotonic saline hydration
b)
Aggressive management of intracranial hypertension.
2)
Systemic anticoagulation
a)
Anticoagulation does not dissolve the thrombus but prevents propagation of the thrombus and reocclusion as the natural fibrinolytic processes take place.
b)
An early randomized trial of 20 patients and retrospective study demonstrated significantly better outcomes in CVT patients treated with heparin, even in the presence of intracerebral haemorrhage.206
c)
A retrospective study of 79 cases of CVT treated with heparin showed a mortality rate of 10% despite anticoagulation207
d)
Long term anticoagulation is required, since recurrent CVT or other venous thrombosis may occur 3–6 months later in 5.5–26% of these patients.208
e)
A recent prospective, nonrandomized cohort study of 624 patients with CVT found that low-molecular weight heparin had better efficacy and safety than unfractionated heparin for the initial treatment of CVT.209
3)
European Federation of Neurological Societies recommendations:210
a)
Oral anticoagulation for 3 months
b)
Three months oral anticoagulation if CVT due to transient risk factor.
c)
Six to 12 months if idiopathic or mild thrombophilia such as heterozygous factor V Leiden or prothrombin G20210A mutation.
d)
Anticoagulation for an indefinite period for patients with recurrent CVT or with “severe” thrombophilia like prothrombin, protein C or S deficiency, homozygous factor V Leiden, or prothrombin G20210A, antiphospholipid antibodies, or a combination of thrombophilias.
4)
Get Clinical Tree app for offline access
Endovascular management
a)
Endovascular treatment is indicated for patients unable to receive anticoagulation and for those who deteriorate despite anticoagulation heparin.
i)
Consider endovascular therapy for patients in high-risk categories including those with seizures, coma, disturbed consciousness, deep cerebral vein thrombosis, posterior fossa involvement, and/or progressive focal deficits.175
b)
c)