div class=”ChapterContextInformation”>
15. Vessel Wall Imaging in the Era of Artificial Intelligence
Keywords
Artificial intelligenceDeep learningVessel wall imagingPlaque analysisRisk predictionIntroduction
Artificial intelligence (AI) in medicine typically uses advanced computational algorithms to perform diagnosis comparable to doctors or domain experts, by mathematically modeling human intelligence, or neural structure or activities. It is beginning to be widely used in several fields of radiology such as in cancer, neuro and cardiovascular.
In this chapter, we will discuss AI methods that can learn and establish data-driven models particularly using deep neural networks to accomplish various imaging and analysis tasks, such as imaging acceleration, image quality enhancement, and image analysis for vessel wall imaging (VWI).
Vessel wall of arteries can be imaged by only a few imaging modalities since it requires high spatial resolution and high contrast to distinguish the vessel wall from surrounding tissues. So far, magnetic resonance imaging (MRI) is the most comprehensive noninvasive modality for VWI in various vasculatures [1, 2]. Ultrasound can also allow for noninvasive vessel wall images but only in certain locations such as in the carotid arteries. In addition, invasive imaging from the luminal side of vessels is possible with intravascular ultrasound or optical coherence tomography. However, these imaging schemes are typically limited to some short segments of the arteries at specific locations. Therefore, the opportunities for AI applications are greater for MRI and ultrasound owing to the larger number of datasets available for training.
Currently, the role of AI in VWI is still in its infancy. We will briefly discuss several examples of AI-based techniques for VWI in two aspects. From vessel wall MRI acquisition perspective, AI methods can provide new opportunities for image acquisition optimization to improve the image quality and reduce acquisition time. On the other hand, AI methods can provide more accurate and clinically oriented post-processing tools to offer various pathways for MRI and ultrasound vessel wall image analysis.
Vessel Wall MRI Acquisition
Vessel wall MRI usually requires high-resolution, large coverage, and black-blood (BB) imaging features in sequences design to meet the clinical needs for atherosclerotic plaque detection. High-resolution is required to accurately evaluate the vessel wall condition and stratify the disease stages. Atherosclerosis is a systemic disease of vessel walls. Therefore, screening the entire vascular bed requires a large coverage imaging scheme. Furthermore, BB imaging techniques ensure good image contrast between lumen and vessel wall, and enable more accurate plaque burden measurements. However, these imaging requirements increase the challenges for obtaining diagnostic image quality for clinical application. To achieve high-resolution imaging, there are inevitable signal-to-noise ratio (SNR) penalties during data acquisition. This SNR loss will be further aggravated when large coverage BB imaging techniques [3, 4] are applied. In addition, the high-resolution large coverage MRI scan requires a long data acquisition time that might pose further challenges in clinical settings, as some patients may have difficulties to keep still for a long time which may further degrade the image quality due to motion. In this section, we discuss the application of AI methods to enhance image quality and accelerate imaging acquisition.
Adequate SNR level and image sharpness are essential factors to detect the presence of small plaques and ensure the accuracy of morphological measurements such as vessel wall thickness and plaque burden. AI methods can retrospectively enhance the image quality in several aspects, including reduction of noise and artifact levels and improvement of image sharpness. These requirements for retrospective image quality enhancement are closely related to the state-of-the-art image restoration problems, such as image denoising and super-resolution. With the recent advent of supervised deep learning methods, properly trained deep convolutional neural networks (CNN) can further boost the image restoration performance while significantly reducing the processing time by using the more advanced graphic processing unit (GPU) hardware. In the image denoising task, the deep CNN approach can outperform other competing methods by 0.2 dB to 0.6 dB of Peak SNR (PSNR) measurements [5]. Also, this trained denoising CNN model has demonstrated good generalization capability to tackle several other image restoration tasks. In addition, some recent studies [6–8] have explored efficient neural network architectures and effective optimization objectives for single image super-resolution task to achieve 0.9–1.1 dB PSNR improvements on more accurate estimation of structural boundaries and detailed textures compared to conventional methods. However, application of these new AI-based image post-processing methods to vessel wall image quality enhancement requires further investigation to demonstrate its clinical value and benefits.
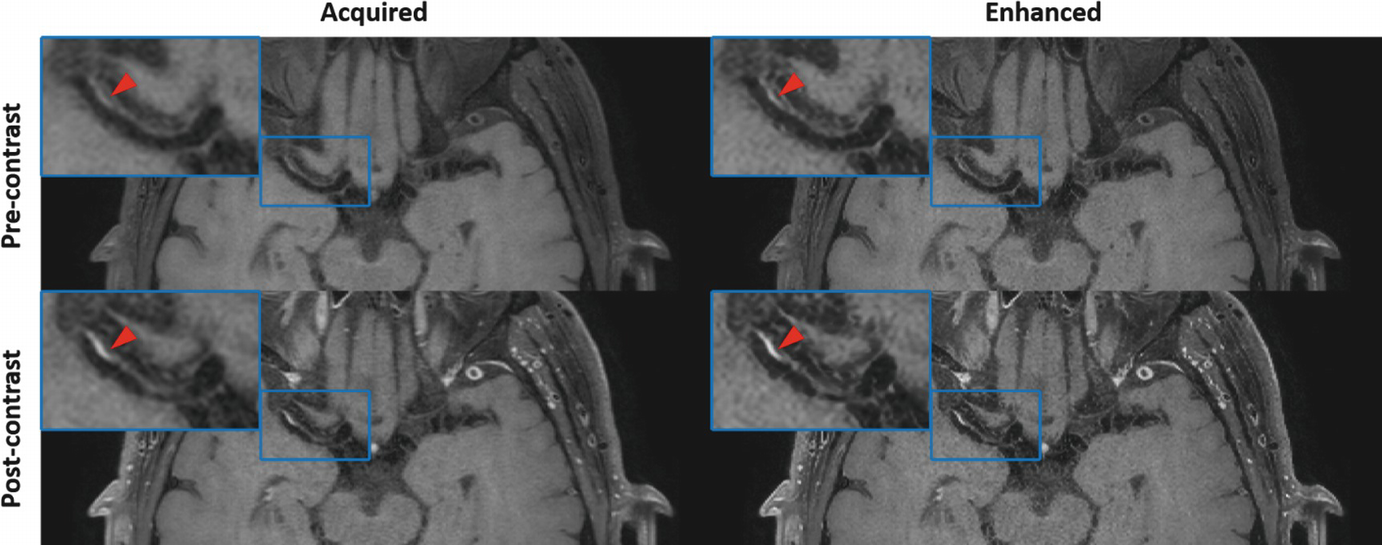
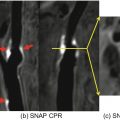
An example of convolutional neural network (CNN)-based image enhancement from a patient acquired with optimized 3D T1-weighted TSE sequence. For both pre- and post-contrast images, CNN-enhanced results can provide clearer definition of the intracranial vessel wall and atherosclerotic plaque (as shown by red arrows) at the right middle cerebral artery
AI methods can also play an important role in imaging acceleration and help reduce the scan time and potential motion-related problems. One approach relies on deep learning-based super-resolution technique to estimate unacquired high-frequency components from the fully sampled low-frequency k-space and obtain a spatially interpolated image with an improved resolution. A recent work [13] demonstrated the feasibility of high-resolution image retrieval from its 4× downsampled image by using a 3D densely connected neural network model for intracranial vessel wall imaging that can result in 4-fold acceleration. The other approach to achieving imaging acceleration relies on the compressed sensing theory. A tailored deep neural network can represent the image in a more appropriate transformed sparse domain, so that the partially incoherent sampled k-space can more accurately restore the artifact free image than using universal or learned sparse transforms with shallow architectures [14–17]. In comparison to traditional imaging acceleration methods, deep learning-based image reconstruction approach may better preserve the natural appearance of MR images and, thus, pathologies. Compared to traditional image reconstruction, AI-based reconstruction has significantly reduced image reconstruction times thereby enabling an easier translation of this technique into the existing clinical workflow.
Vessel Wall Imaging Analysis
As described in the chapter for vessel wall image analysis, VWI analysis can be used for many purposes from screening to full comprehensive vascular analysis, to diagnose different vascular pathologies, and it can be applied in various different imaging modalities. AI methods may play important roles in all aspects of analysis.
MRI Vessel Wall Analysis
VWI and analysis are important for the differential diagnosis of diseases such as vasculitis, atherosclerosis, and arteriosclerosis. We will discuss atherosclerotic plaque analysis in detail in this section.
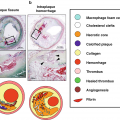
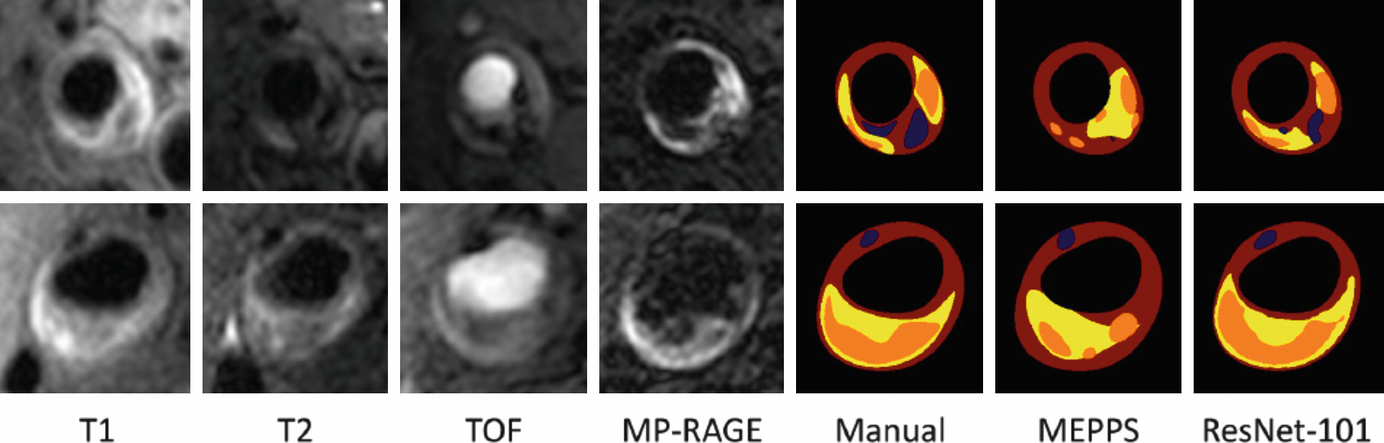
Both identification and quantification of plaque components have been shown to predict clinical outcomes. Plaque components such as lipid-rich necrotic core (LRNC), calcification, intraplaque hemorrhage (IPH), loose matrix, fibrous cap, and fibrous tissue can be identified using vessel wall MRI. The presence of intraplaque hemorrhage in the carotid arteries is associated with stroke or transient ischemic attacks in the ipsilateral side [18]. Plaques with large LRNC, IPH, and a disrupted fibrous cap are deemed to be at higher risk for events [19, 20]. Moreover, plaque composition is also known to affect the progression of disease. For example, the presence of IPH is known to stimulate plaque progression in the same artery [21]. Quantitative measurements of the vessel wall were instrumental in describing such clinical associations. Combination of qualitative and quantitative information from plaque analysis form the basis of risks scores [19].
VWI provides a rich but complex set of images particularly in the case of MRI where multiple contrast weightings of the same anatomical location are produced. Information from these image weightings has to be combined for interpretation of the vessel wall composition. While such interpretation is common in radiology, the challenges for this application are the small size and variable plaque composition within a small region of plaque. While manual review is workable for plaque component identification, quantification requires segmentation from multiple image slices and leads to longer review time, increased inter-reader variability and intra-reader variability. AI is well suited to reducing the labor and measurement errors in quantification. Automatic segmentation methods can provide reproducible measurements, while human observers could check the accuracy of component identification and further train the models for improved performance.
An additional challenge in VWI is caused by the fact that VWI interpretation is not yet routine clinical practice. Therefore vessel wall analysis requires intensive training at selected centers. These factors also reduce the opportunity for more wide-ranging use of this technology. Once trained and validated, AI systems may provide the opportunity for more medical centers to bring the benefits of plaque imaging to their patients.
Automatic plaque component identification and quantification requires several processing steps. The essential step for quantification is plaque segmentation where each image pixel is classified into a plaque component label. However, there are other steps that may also be required depending upon the imaging modality and anatomical location. If multi-contrast MRI and/or multiple imaging time points need quantification, then image registration will be required. Complex arterial geometries also require additional efforts to identify and localize the plaque region to be quantified.
Plaque Segmentation

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree