Fig. 23.1
(a) Schematic view of a simplistic workflow model of imaging infrastructure by separating the workflow of diagnostic procedures blue arrows and image-guided surgery procedure red arrows. (b) MedModel simulation of workflow. In a first attempt of modeling and simulation, we have been trying MedModel, a software for the purpose of workflow simulation in hospitals outpatient units. The initial results are shown in (b). At that time the software was not sufficient in the required details of micro-ergonomics, but the macro-ergonomic assessment of equipment utilization was helpful to improve the design of the imaging systems infrastructure. A first implementation of this approach in clinical settings is currently taking place at the University of Dundee, Scotland, by combining 3-T MRI and a 128 multi-slice PET/CT with a multifunctional interventional/surgical suite MITOS (see Fig. 23.2). The focus is on the development of multimodal image-guided diagnostic and therapeutic procedure for early detection and treatment of cancer and cardiovascular diseases [7]
In a first attempt of modeling and simulation, we have been trying MedModel, a software for the purpose of workflow simulation in hospital’s outpatient units [8]. The initial results are shown in Fig. 23.1b. At that time the software was not sufficient in the required details of micro-ergonomics, but the macro-ergonomic assessment of equipment utilization was helpful to improve the design of the imaging systems infrastructure. A first implementation of this approach in clinical settings is currently taking place at the University of Dundee, Scotland, by combining 3-T MRI and a 128-multi-slice PET/CT with a multifunctional interventional/surgical suite MITOS (see Fig. 23.2). The focus is on the development of multimodal image-guided diagnostic and therapeutic procedure for early detection and treatment of cancer and cardiovascular diseases [7].
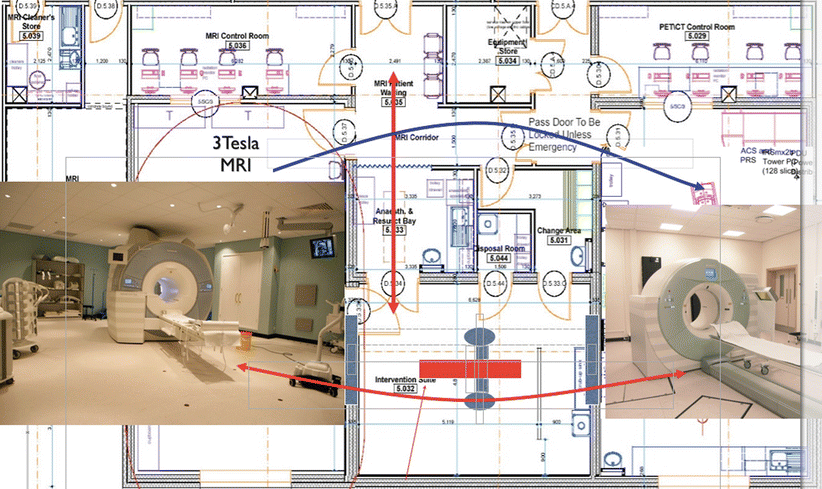

Fig. 23.2
MITOS integrated multimodality image-guided diagnosis and therapy set up at the Clinical Research Center of University Dundee and NHS Tayside, Scotland, (3-T interventional MR and 128-multi-slice interventional PET/CT interconnected with a multipurpose interventional suite (diagnostic workflow: blue arrows, image-guided procedure workflow: red arrows)
Jolesz et al. have developed a similar concept named AMIGO for integration of MRI and PET/CT with a surgical suite (installed at BWH, Harvard Medical School, Boston, MA, USA) (see Chap. 24). At the new International Clinical Research Center in Brno (Czech Republic), novel image-guided procedures for MR-guided cardiology are being developed in cooperation with IIIOS-MITOS research in Dundee and in collaboration with Mayo Clinics, Rochester, MN, USA [9].
Mathematic modeling and simulation appears to be an effective technique for the understanding of image-guided processes and to analyze, design, and evaluate different alternatives and configurations within the same environment. As a result and facing the growth of patient numbers, some centers are using computer simulation to increase the capacity to match the demand without dramatic investment, or even implementing real-time decision-support systems, used also as predictors of emergency scenarios [10, 11]. Also, in the case of surgical environments, computer simulation has helped to increase the efficiency and utilization of ORs, becoming a useful tool for enhancing the scheduling, planning, and workflow in surgical units [12, 13]. There is however a lack of workflow analysis and optimization for image-guided therapy and integration of multimodality imaging with surgical/interventional procedures.
Overview of Modeling Techniques in Healthcare Systems
In the review conducted by England and Roberts [1], the authors also reported the most common methods applied in modeling healthcare environments: regression, econometric, mathematical modeling employing queuing theory or stochastic methods, and mathematical programming. More recently, Sobolev et al. [2] distinguish between static and dynamic approaches, deterministic or stochastic, and methods that involve discrete or continuous time. Among these techniques, Monte Carlo models, as static simulation methods, and Markov chains and discrete event computer simulation (DES) models, as dynamic approaches, are the most commonly used for clinical systems. In other cases, Petri nets were used for the modeling [14, 15].
Monte Carlo (MC) models consist of a random repetition of samples with probabilities, representing the process outcome at a particular point in time [2], hence the reference to the famous Monte Carlo Casino in Monaco and its gambling games. There are different methods applying Monte Carlo: classical, quantum, integral simulation, and so on. [16] Within the healthcare context, MCs are used mainly for risk assessment, prognostic and transmission models of health interventions, and cost-benefit analysis of medical treatments, among others [17].
Markov chains (MKC) are discrete event stochastic models, where the states are defined as nodes and transitions between the states are represented by links. Figure 23.3 shows the probability (P 0,1) to reach from 0 to 1, and (P 1,0) is the probability to turn back to 0. The probability to continue in the state 1 is P 1,1. Markov chains, despite their usual application with discrete instants of time, can be used with a continuing time line, which means that transitions can occur at any time. One of the main properties is that they do not have memory. Each next state depends only on the current state and not on a sequence of previous events. This property makes MKC not appropriate when a new state may depend not only on the previous state but also on a sequence of states that preceded it, as happens during image-guided therapy. An additional limitation is the impossibility of describing interactions between concurrent processes [2, 18].
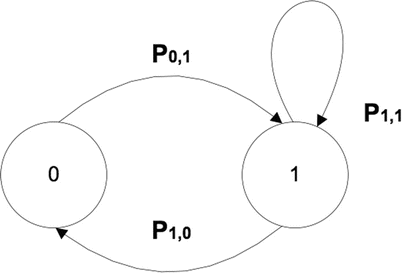

Fig. 23.3
Example of a simple Markov chain of workflow
Petri nets (PNs) define the structure of the system using two elements in their graphs. The graph’s nodes or places represent the system states, and the transitions represent the net evolution. In the example of Fig. 23.4, L1 to L3 are the nodes, and t1 to t2 are the transitions. A PN defines initially a static view of the system. To study the dynamics, the PN has to be executed, and for that reason, a token is placed (black dot in the Fig. 23.4) on one of the nodes. During execution, the token is taken from the input node to every output node (L2 and L3 in the example). Each execution of the transition is called firing the transition [18]. The main drawback with PNs is that they can be very complicated to read for large and complex models as all the data has to be represented in the net. In addition, they do not define hierarchical concepts. Colored PNs can consider these definitions up to a degree.
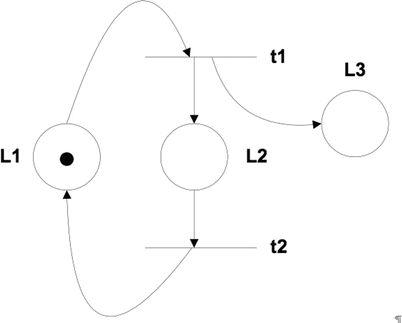

Fig. 23.4
An example of a Petri net graph of system structure
Discrete event computer simulation (DES) consists of a mathematical modeling technique that allows building of hierarchical and modular models including simple as well as complex models. It permits the modeling of systems with a set of infinitive possible states where each new state after an event arrival can depend on previous states. The system to be modeled maintains a “clock,” marking time stamps throughout the event’s duration. The system may also have “buffers,” where components accumulate while awaiting and processing, and “sinks” that allows the part (what is processed) to exit the system. When modeling, data are collected on frequencies of parameters, arrival rates, and process times. This information is then analyzed statistically to determine the distributions that represent the groups of data that will be introduced to the simulation models. In health applications, there normally appear two types of approach. The first, event scheduling, samples the moments when events occur from predefined distributions of times. The second approach, process interaction, describes the chronology of actions associated with the events, modeling the process as a sequence of serial and concurrent activities operating on, what some experts call, passive entities (e.g., patients or clinicians). Therefore, discrete event models are found to be appropriate for modeling workflow in surgery and seem suitable for image-guided therapy [2, 18, 19].
Workflow Modeling and Simulation in Surgical Environments
The demand in the ORs has increased and stimulated applying workflow modeling and simulation to analyze and improve facilities and processes.
One of the critical factors that determine the workflow analysis is the amount and detail of data available. Hospitals usually hold databases with information about the operations performed. These databases are usually different for each department. In most cases, those records are usually limited to just a few metrics of the operating process such as waiting time before operating, surgery time, or recovery time. The majority of these studies are focused on improving the efficiency on using the ORs and reducing waiting lists of patients, where a large amount of data is needed to come to significant results [20, 21]. Some authors have dedicated most of the project period to data collection in order to have enough data to implement realistic models [12]. However, in cases where a more detailed workflow description of the intervention events is needed, it is unusual to find databases with the kind of required information available. Some authors have completed database records by interviewing experts or taking measurements [22]. Other authors introduced new technologies into the ORs to help the data gathering. Nara et al. [23] used an ultrasonic sensor system to localize positions of the staff during neurosurgery operations at the Tokyo Women’s Medical University in Japan. Figure 23.5 shows the setup of the sensors in the operating room and the placement of the transmitters on the clinicians without interfering in their work.
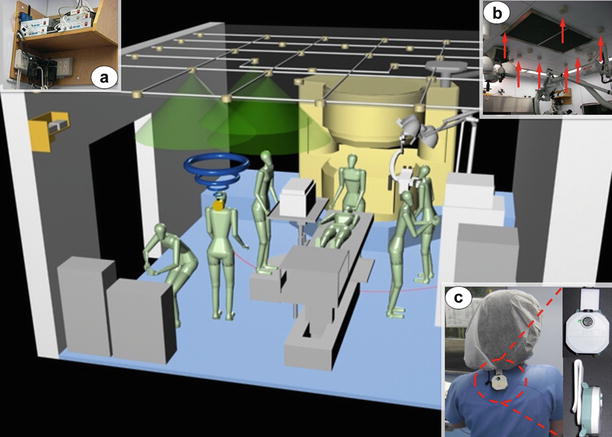

Fig. 23.5
Ultrasonic 3D location aware system at Tokyo Women’s Medical University (Japan), consisting on control units (a), receivers (b) and transmitters (c) [23]
Padoy et al. [24] studied the feasibility of introducing a signal-based modeling system able to recognize signals from the different devices used during the laparoscopic cholecystectomies. Thereby each device can be analyzed, by which person and for what purpose it’s being used at discrete time steps. The information was recorded with synchronized video cameras and presented as statistical modeling for the signal and phases recognition. Obtaining sufficient information for optimal reengineering of OR management requires a systematic framework for collecting data in order to track inefficiencies in the process [15].
Another important factor in workflow modeling is the type of intervention that is being analyzed. Emergency surgery cannot be scheduled in advance; therefore, other types of data are taken into account to improve workflow in the operating rooms. In some studies, such as in Torkki et al. [20] reorganized the flow of patients and also the guidance of the process, redistributing tasks among the clinicians and moving phases of the operation to decrease waiting times. On the other hand, some surgical procedures have a high variability in their requirements like open cardiac surgery with an average duration of 4–5 h. Using the hospital database to get records from years 2001 to 2003, Peltokorpi et al. [25] evaluated three different process changes for open heart surgery: cost analysis and underused and overused time scheduled for the surgery in the OR. The authors agreed that for a more accurate modeling to predict OR usage, the data in the hospital were limited and a specific project would be required. Despite the limitations that appear in many studies, the effort made towards better modeling of workflow in ORs has been extended successfully to many disciplines such as cataract surgery [26], trauma surgery, [13, 20] endoscopy [12], laparoscopy [24], and also radiology but not yet to interventional procedures and image-guided therapy.
Workflow Modeling and Simulation in Radiology
In 1971, Garfinkel [27] published a study about applications of computer simulation to improve patient scheduling in health systems, which included radiology as one of the highlighted fields. A year later, Jeans et al. [28] presented a work about simulation on an X-ray department in Bristol (UK). The authors studied and modeled the workflow describing human resources, equipment, arrival and waiting times, types of examinations, and overall time that patients spent in the department. Their simulations evaluated workload and resources used in order to predict probable improvements achieved by trying different alternatives. In 1981, O’Kane [29] provided another simulation model for an X-ray department, introducing some new factors in the modeling such as staff breaks. The model distinguishes the time of examination and the time in which the rooms or radiographers would be available for the next patient, taking into account that an examination can be finished but the room might need some work before other patients can use it and that radiographers have certain duties regarding dealing with records and checking images. Lev et al. [30] developed a similar workflow model for scheduling process of patients. All these authors agreed that the improvements in radiology should be directed towards the optimal design of the management system and not to reducing the staff or other facilities.
The problem of improving scheduling practices, waiting times, and resource utilization has been dealt with by more refined modeling techniques for workflow simulation such as Markov decision processes and DES [31, 32]. Other authors have focused their work on the integration of information system in radiology departments and hospitals, including the picture archiving and communication systems (PACS) [33, 34]. Lindsköld et al. describe how this technique has the potential to support proper planning and use of personal, space, and equipment resources. This study reveals however that there is a lack of studies that fully explore simulation as a tool to facilitate changes and integration of new standards (DICOM, HL7) and also different imaging technologies (MRI, multi-slice CT, PET/CT, ultrasound) [35].
In addition to the academic environment, global companies are working on products to facilitate and improve workflow in image-guided environments. For example, Siemens (Erlangen, Germany) has developed DotTM (Day optimizing throughput), a software that provides an easy-to-use interface to improve examinations workflow for MRI diagnosis [36]. The company has also designed Symbia.netTM that intends to be a solution for acquisition, processing, and integration of SPECT and CT images to give clinicians access to all patient data for a better diagnostic [37]. In addition, Siemens has created a software platform, Tecnomatix Plant Design and Optimization, for plant design and optimization through discrete event simulation that, although it appears to be designed for manufacturing industry, it has been used successfully for modeling a radiology department [32, 38]. Other companies like Philips Einthoven, Netherlands, GE Healthcare (Milwaukee, IL, USA), or Dräger (Lübeck, Germany) provide support for room layouts and ergonomics of operating rooms and integration of imaging modalities to their customers to help hospital managers and clinicians to find the right solution for their needs [39–41]. Despite the fact that these activities increasingly cover diagnostic imaging procedures, the urgent need for workflow analysis and optimization of complex image-guided therapy has not yet been established. In the following section, we described the first attempt in this direction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree