Chapter 100 Overview: Duplication cysts may occur anywhere along the gastrointestinal tract, from the mouth to the rectum. They typically occur on the mesenteric surface of the viscus to which they are attached. More than 80% are spherical and do not communicate with the lumen, whereas approximately 18% are tubular and may communicate with the lumen of the adjacent bowel. Duplication cysts are named by the segment of gut to which they are attached, not by the type of mucosa lining the lumen, which may include representatives of all portions of the gastrointestinal tract, including the pancreas.1 Gastric duplication cysts are rare and comprise approximately 4% to 7% of intestinal duplications.1,2 Although the cysts usually are located along the greater curvature of the stomach, they may occur in other regions of the stomach, including the pylorus,3–5 in addition to ectopic sites.6 Most gastric duplication cysts are spherical and typically do not communicate with the gastric lumen. Histologically, the cysts usually are lined with gastric mucosa.2 Ectopic pancreatic tissue has been reported in approximately 37% of cases,1,7,8 with rare reported communication with the pancreatic ductal system.7 Etiology: The etiology remains controversial. Several hypotheses have been postulated to explain the embryology of gastrointestinal tract duplications because no single theory is sufficient to explain the characteristics of all types of duplication cysts. These theories include abnormal luminal recanalization, intrauterine ischemia, abortive twinning, bronchopulmonary foregut malformation, and persistence of an embryologic diverticulum.1,9–13 Clinical Presentation: Gastric duplication cysts occur more frequently in females at a ratio of 2 : 1.14 If it is not discovered prenatally on ultrasound,15,16 postnatal clinical presentation usually occurs during the first year of life17 with symptoms including intestinal obstruction, a palpable mass, gastrointestinal bleeding, abdominal pain, and hematemesis18,19 or melena.20 Uncommon complications of gastric duplication cysts include pancreatitis as a result of ectopic pancreatic tissue with perforation and pseudocyst formation21 and gastric outlet obstruction simulating hypertrophic pyloric stenosis.4,22,23 Imaging: Although gastric duplication cysts may be evident on plain radiography as a masslike density in the upper abdomen,18 the low sensitivity and specificity limits the utility of plain radiography. Gastrointestinal contrast examination may demonstrate an effect of the mass on the adjacent hollow viscus by the cyst (Fig. 100-1), and uncommonly it may demonstrate communication between the stomach and the cyst. Ultrasonography using a high-frequency transducer is an excellent imaging modality for characterization of these cystic masses, which often demonstrate the “gut signature” or “double-wall” sign, representing the inner hyperechoic mucosal layer and peripheral hypoechoic muscle layers typical of the gastrointestinal tract (Figs. 100-1, B, and 100-2); free fluid may be found in cases of perforation (Fig. 100-3). Cysts may be completely anechoic or may contain septations and internal echoes representing proteinaceous material, blood products, or debris related to infection24 (see Fig. 100-2). Computed tomography (CT) demonstrates a well-circumscribed homogeneously low-attenuating mass in the left upper quadrant. Peripheral enhancement, wall thickening, and heterogeneous attenuation of the cyst wall may be present if complicated by inflammation. On magnetic resonance imaging (MRI), the cysts exhibit fluid characteristics at T1 and T2 weighting, but the signal may increase on T1-weighted sequences in the presence of hemorrhage or other proteinaceous material. Imaging with technetium 99m–pertechnetate is very helpful when gastric mucosa lines the cysts.25 Meticulous technique is necessary to differentiate the duplication cyst from the adjacent stomach; multiple views may be required. Single photon emission tomography CT may be useful in defining the abnormality. Figure 100-1 Gastric duplication in a 6-week-old infant with hematemesis. Figure 100-2 Gastric duplication cyst. Figure 100-3 A ruptured gastric duplication cyst. Treatment: Treatment of both symptomatic and asymptomatic gastric duplication cysts entails surgery with complete excision of the cyst. If the cyst cannot be resected without violating the adjacent gastric lumen, a partial gastric resection may be necessary.7 Laparoscopic resection of gastric duplication cysts also has been described.26–28 Overview: Gastric diverticula are rare in the general population, with a reported prevalence of 0.02% to 0.04%; they are even less common in children, with 4% of gastric diverticula occurring in patients younger than 20 years.29,30 Gastric diverticula may be congenital or acquired; the congenital form constitutes approximately 72% of all gastric diverticula. Congenital gastric diverticula are true diverticula, containing all three layers of the gastric wall. These diverticula typically involve the posterior wall of the stomach within 2 to 3 cm of the gastroesophageal junction. Acquired gastric diverticula are pseudodiverticula and usually are located near the gastric antrum.29 Etiology: The cause of congenital gastric diverticula is thought to be related to the division of the longitudinal fibers of the wall of the stomach in such a way that results in a defect in the musculature of the gastric wall, which is affected by arterial perforators and the absence of peritoneal investment of the posterior gastric wall, which contribute to the weakness of the gastric wall.29 Acquired diverticula, on the other hand, usually are related to peptic ulcer disease or pancreatitis.29 Clinical Presentation: Although most gastric diverticula are clinically asymptomatic, symptomatic lesions in children tend to present in early childhood and adolescence. The symptoms can range from nonspecific complaints such as recurrent abdominal pain, nausea, and vomiting to massive hemorrhage and perforation; such symptoms may relate to the presence of ectopic pancreatic tissue within the diverticula, causing erosions and ulceration.29,31–33 Large antral partial diverticula can cause partial gastric outlet obstruction by compression or intussusception of the antropyloric region (Fig. 100-4). Imaging: Imaging evaluation of suspected gastric diverticula includes a fluoroscopic contrast upper gastrointestinal series, which demonstrates an outpouching of the gastric wall that communicates with the gastric lumen. Right anterior oblique positioning optimizes the sensitivity of this imaging study because of the posterior wall location of most congenital gastric diverticula. Gastric diverticula also may be identified with cross-sectional imaging. Overview: Congenital microgastria is a rare anomaly of the pediatric gastrointestinal tract characterized by a small, tubular stomach, typically associated with a distended esophagus. In persons with microgastria the stomach is lined by normal gastric mucosa but the total cell mass is significantly reduced.35,36 Agastria, or complete absence of the stomach, is the most extreme form of microgastria. Congenital microgastria is almost always accompanied by other congenital anomalies; isolated congenital microgastria is extremely rare. Associated congenital anomalies include a wide range of entities such as heterotaxy with asplenia and malrotation,37 congenital diaphragmatic hernia,38 tracheoesophageal clefts, hiatal hernia,36 jaw and palate abnormalities,39 DiGeorge syndrome, primary ciliary dyskinesia,40 central nervous system abnormalities, and vertebral, cardiac, renal, and limb reduction anomalies (VACTERL association). It has been suggested that microgastria in association with limb reduction defects and central nervous system anomalies may have an autosomal recessive mode of inheritance.41,42
Congenital and Neonatal Abnormalities
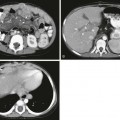
Gastric Duplication Cyst

A, Barium enema study demonstrates a mass (M) between the greater curvature of the stomach and the contrast-filled transverse colon. B, An ultrasound study demonstrates typical findings of fluid-filled cyst (C). The stomach (S) contains shadowing gas. The connection of the cyst with the gas-filled stomach is seen (arrow). C, An upper gastrointestinal study performed after barium enema administration demonstrates mass effect (M) on the greater curvature of the stomach. (Courtesy Dr. D. Barlev, New York, NY.)

An ultrasound image of the upper mid abdomen along the long axis of the body and antrum of the stomach demonstrates a complex cystic mass contiguous with the greater curvature of the stomach, with a hypoechoic wall suggesting the muscular layer of gut signature.

A sagittal ultrasound image through the medial portion of the right lobe of the liver (L) demonstrates a thin-walled, approximately 4-cm simple cyst (asterisk). Free fluid, complex in its dependent portion, was found at surgery to represent hemorrhage resulting from rupture. (Courtesy Dr. C. Mitchell, Peoria, IL.)
Gastric Diverticula
Congenital Microgastria