Mediator
Characteristics and role in inflammation
A. Cell factors
Histamine
Stored in mast cells, basophil and eosinophil leukocytes, and platelets
Release from sites of storage is stimulated by complement components C3a and C5a and by lysosomal proteins released from neutrophils
Responsible for vasodilation and the immediate phase of increased vascular permeability
Lysosomal compound
Released from neutrophils and includes cationic proteins, which may increase vascular permeability, and neutral proteases, which may activate complement
Prostaglandins
Long-chain fatty acids derived from arachidonic acid and synthesized by many cell types. Some prostaglandins potentiate the increase in vascular permeability caused by other compounds
Leukotrienes
Synthesized from arachidonic acid, especially in neutrophils, and have vasoactive properties
5-Hydroxytryptamine (serotonin)
A potent vasoconstrictor present in high concentrations in mast cells and platelets
Lymphokines
Released by lymphocytes and may have vasoactive or chemotactic effects
B. Plasma factors
Products of complement activation
C5a
Chemotactic for neutrophils, increases vascular permeability, releases histamine from mast cells
C3a
Similar to but less active than C5a
C567
Chemotactic for neutrophils
C56789
Cytolytic activity
C4b, 2a, 3b
Facilitates phagocytosis of bacteria by macrophages (opsonization of bacteria)
Kinin system
Bradykinin included in the system is the most important vascular permeability factor, also a mediator for pain which is a major feature of acute inflammation
Coagulation factors
Responsible for the conversion of soluble fibrinogen into fibrin, a major component of the acute inflammatory exudate
Fibrinolytic system
Plasmin included in the fibrinolytic system is responsible for the lysis of fibrin into fibrin degradation products, which have a local effect on vascular permeability
Acute inflammation is characterized by the following major regional components:
Local Vascular Changes
1.
Vasodilation following transient vasoconstriction is one of the most important changes that accompany acute inflammation, and it persists until the end of the process. It involves first the arterioles and then results in the opening of new capillary beds in the area.
2.
Increased vascular permeability due to:
Contraction of endothelial cells with widening of intercellular gaps
Direct endothelial injury, resulting in endothelial cell necrosis and detachment
Leukocyte-mediated endothelial injury: Leukocytes adhere to the endothelium, which becomes activated, thereby releasing toxic oxygen species and proteolytic enzymes and causing endothelial injury.
Angiogenesis: With inflammation, endothelial cells may proliferate and form new capillaries and venular beds (angiogenesis). These capillary sprouts remain leaky until endothelial cells differentiate.
3.
Stasis (slowing of circulation): Increased permeability with extravasation of fluid into the extravascular spaces results in concentration of red blood cells in the small vessels and increased viscosity of the blood, with slowing of circulation in the local vessels. Figures 4.1 and 4.2 illustrate the main vascular changes.
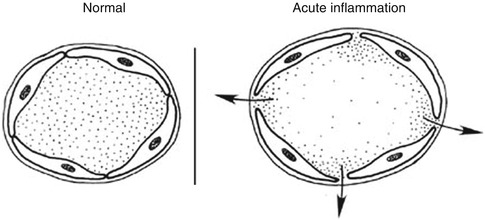
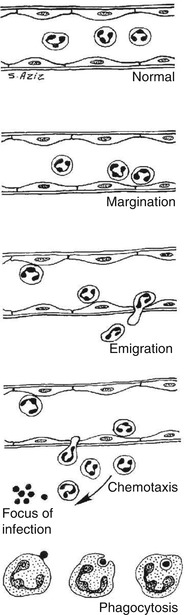

Fig. 4.1
Vasodilation of vessels and opening of the intercellular gaps in inflammation

Fig. 4.2
Sequence of cellular changes that accompany inflammation
Formation of Exudate
Increased permeability of the microvasculature, along with the other changes described, leads to leakage with formation of “exudate,” an inflammatory extravascular fluid with a high protein content, much cellular debris, and a specific gravity above 1.020. This is the hallmark of acute inflammation, which may also be called exudative inflammation. It indicates significant alteration in the normal permeability of the small blood vessels in the region of injury.
The two components of exudate, fluid and protein, serve good purposes. Fluid increase helps to dilute the toxins. Protein increase includes globulins that provide protective antibodies, while fibrin helps to limit the spread of bacteria and promotes healing. Exudate varies in composition. In early or mild inflammation, it may be watery (serous exudate) with low plasma protein content and few leukocytes. In more advanced inflammation, the exudate becomes thick and clotted (fibrinous exudate). When large numbers of leukocytes accumulate (Fig. 4.3), the exudate consists of pus and is called suppurative, while if it contains erythrocytes due to bleeding, it is referred to as hemorrhagic. Pus, accordingly, is a variant of exudate that is particularly rich in leukocytes, mostly neutrophils and parenchymal cell debris.
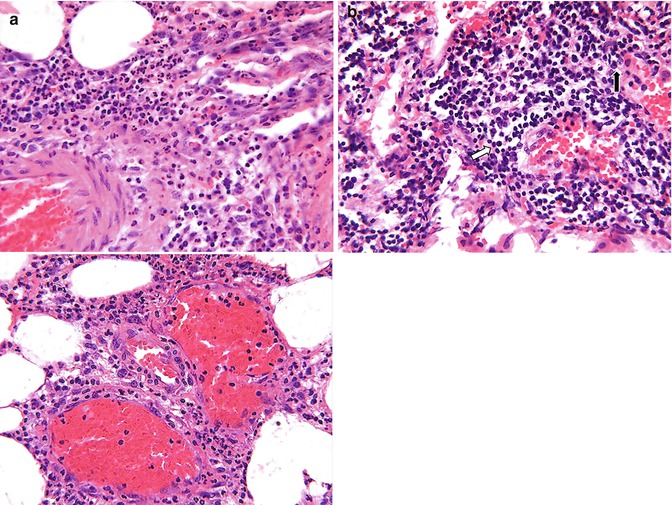

Fig. 4.3
(a) Microphotograph of acute inflammation showing numerous inflammatory cells particularly polymorphonuclear leukocytes, which are identified better (arrows) on higher power. (b) Microphotograph of chronic inflammation illustrating the different types of inflammatory cells, the mononuclear cells including lymphocytes (arrow) and plasma cells (open arrow)
Local Cellular Events
1.
Margination
After stasis develops, leukocytes will be peripherally oriented along the vascular endothelium, a process called leukocytic margination (Fig. 4.1).
2.
Diapedesis (emigration)
Leukocytes emigrate from the microcirculation and accumulate at the site of injury.
3.
Chemotaxis
Once outside the blood vessel, the cells migrate at varying rates of speed in interstitial tissue toward a chemotactic stimulus in the inflammatory focus. Through chemoreceptors at multiple locations on their plasma membranes, the cells are able to detect where the highest concentrations of chemotactic factors are and to migrate in their direction. Granulocytes, including the eosinophils, basophils, and some lymphocytes, respond to such stimuli and aggregate at the site of inflammation. The primary chemotactic factors include bacterial products, complement components C5a and C3a, kallikrein and plasminogen activators, products of fibrin degradation, prostaglandins, and fibrinopeptides. Histamine is not a chemotactic factor but facilitates the process. Some bacterial toxins, particularly from gram-negative bacteria and streptococcal streptolysins, inhibit neutrophil chemotaxis [2, 3, 5].
4.
Phagocytosis
This defense mechanism is particularly important in bacterial infections. The polymorphonuclear leukocytes and macrophages ingest debris and foreign particles.
4.3.1.2 Local Sequelae of Acute Inflammation
Acute inflammation has several possible local sequelae. These include resolution, suppuration (formation of pus), organization, and progression to chronic inflammation. Resolution means complete restoration of tissues to normal. Organization of tissues refers to their replacement by granulation tissue with formation of large amounts of fibrin, growth of new capillaries into fibrin, migration of macrophages into the zone, and proliferation of fibroblasts resulting in fibrosis and consequently exudate becoming organized.
4.3.1.3 Chronic Inflammation
Acute inflammation may progress to a chronic form characterized by reduction of the number of polymorphonuclear leukocytes but proliferation of fibroblasts with collagen production. Commonly, chronic inflammation may be primary with no preceding acute inflammatory reaction. Chronic inflammation, whether following acute inflammation or not, is characterized by a proliferative (fibroblastic) rather than an exudative response with predominantly mononuclear cell infiltration (macrophages, lymphocytes, and plasma cells) (Fig. 4.3b). Vascular permeability is also abnormal, but to a lesser extent than in acute inflammation with formation of new capillaries.
4.3.1.4 Abscess Formation
Abscess is defined as a collection of pus in tissues, organs, or confined spaces, usually caused by bacterial infection. The first phase of abscess formation is cellulitis, characterized by hyperemia, leukocytosis, and edema, without cellular necrosis or suppuration. This stage is also called phlegmon. It may be followed in some organisms by necrosis and liquefaction and walling off of the pus, which results in abscess formation that can be present with both acute and chronic inflammation.
4.3.2 Systemic Pathophysiological Changes of Inflammation
Three major systemic changes are associated with inflammation: leukocytosis, fever, and an increase in plasma proteins. Leukocytosis is an increased production of leukocytes due to stimulation by several products of inflammation such as complement component C3a and colony-stimulating factors. A febrile response is due to the pyrogens. The increase in plasma proteins is due to the stimulation of the liver by some products of inflammation, leading to increased synthesis of certain proteins referred to as acute-phase reactants which include C-reactive protein, fibrinogen, and haptoglobin and are anti-inflammatory [2].
4.3.3 Pathophysiological Changes of Healing
Healing of tissue after injury is closely linked to inflammation since it starts by acute inflammation. Healing may lead to restoration of normal structure and function of the injured tissue (resolution) or to the formation of a scar consisting of collagen (repair) when resolution cannot be achieved because the tissue is severely injured or cannot regenerate.
In either case, acute inflammation occurs first and for this reason is considered the defensive phase of healing. Healing (resolution and repair) occurs in two overlapping phases, reconstruction and maturation, and may take as long as 2 years. The reconstructive phase starts 3–4 days after injury, continues for approximately 2 weeks, and is characterized by fibroblasts followed by collagen synthesis. The maturation phase is characterized by cell differentiation, scar formation, and remodeling of the scar; it begins several weeks after injury and may take up to 2 years to complete.
4.4 Pathophysiology of Major Soft Tissue Inflammation
4.4.1 Abdominal Inflammation
An abdominal abscess may be formed in an abdominal organ or within the abdomen outside the organs. There are several types of abdominal infection: abscess; cellulitis (phlegmon), i.e., early inflammation of the soft tissue prior to or without formation of an abscess; and peritonitis. Abscesses fall into three categories:
1.
Intraperitoneal abscess
Subphrenic
Midabdominal
Right lower quadrant
Left lower quadrant
Pelvic abscess
2.
Retroperitoneal abscess
Anterior retroperitoneal
Perinephric
3.
Visceral abscess
Hepatic
Pancreatic
Splenic
The organisms causing abscesses may reach the tissue by direct implantation such as penetrating trauma, may spread from contiguous infection, through hematogenous or lymphatic routes from a distant site, or through migration of resistant flora into an adjacent, normally sterile area such as in perforation of an abdominal viscus.
Factors predisposing to abscess formation include impaired host defense mechanisms; trauma/surgery; obstruction of urinary, biliary, or respiratory passages; foreign bodies; chemical or immunological irritation; and ischemia. Abdominal surgery (particularly of the colon, appendix, and biliary tree) and trauma are the most common; less common are appendicitis, diverticulitis, and pelvic inflammatory disease. The formation of fibrin in the abdominal cavity is a common pathophysiological pathway for abdominal abscess formation due to diminished fibrin degradation. Hyaluronan-based agents were found to reduce adhesion formation after surgery and reduce abscess formation in experimental peritonitis. Possible mechanisms of action of hyaluronan include modulation of the inflammatory response and enhanced fibrinolysis [7]. Low pH, large bacterial inocula, poor perfusion, the presence of hemoglobin, and large amounts of fibrin (which impedes antibiotic penetration) make the abscess a cloistered environment that is penetrated poorly by many antimicrobial therapies [8, 9]. Therefore, management of these infections requires prompt recognition, early localization, and effective drainage, as well as appropriate antimicrobial use. Once the diagnosis is made and the abscess is localized, treatment should begin promptly. Percutaneous or open surgical drainage should be used. Broad-spectrum antibiotics should be given until culture and sensitivity data are obtained. Localization is crucial since, for example, percutaneous drainage is inappropriate for abscesses in certain locations such as the posterior subphrenic space or in the porta hepatis. In the liver, abscesses occur in the right lobe in approximately 95 % of the cases, and in 70 % of cases, the liver abscesses are solitary [10].
Accumulation of leukocytes in the abscess is the pathophysiological basis for using labeled white blood cells for abscess imaging. In the acute phase, migration of leukocytes is vigorous. Later, the migration rate slows, and the cell type changes from predominantly neutrophils to mononuclear cells (lymphocytes, plasma cells, and macrophages). This pathophysiological change associated with the chronic state explains the better diagnostic accuracy of labeled leukocyte scans in acute as opposed to chronic abscesses.
Inflammatory bowel disease (IBD) is an idiopathic disease, probably involving an immune reaction of the body to its own intestinal tract. The two major types of IBD are ulcerative colitis (UC) and Crohn’s disease (CD). Crohn’s disease is also referred to as regional enteritis, terminal ileitis, or granulomatous ileocolitis. IBD is a disease of industrialized nations and observed most commonly in Northern Europe and North America. Incidence among whites is approximately four times that of other races, slightly greater in females and higher in Ashkenazi Jews (those who have immigrated from Northern Europe) than in other groups. The risk of developing UC is higher in nonsmokers and former smokers than in current smokers. Incidence peaks in the second and third decades of life. A second smaller peak occurs in patients aged 55–65 years. CD and UC can occur in childhood, although the incidence is much lower in children younger than 15 years with some differences in presentation and more negative effect on quality of life in younger age group [11].
The etiology of IBD is unsettled. Suspected factors include environmental, infectious, genetic, autoimmune, and host factors. A great deal of research has been performed to discover potential genes linked to IBD. One of the early linkages discovered was on chromosome 16 (IBD1 gene), which led to the identification of the NOD2 gene (now called CARD15) as the first gene clearly associated with IBD (as a susceptibility gene for Crohn’s disease). Studies have also provided strong support for IBD susceptibility genes on chromosomes 5 (5q31) and 6 (6p21 and 19p). None of these mechanisms have been implicated as the primary cause, but they are postulated as potential causes. The lymphocyte population in persons with IBD is polyclonal, making the search for a single precipitating cause difficult. The trigger for the activation of the immune response has not been defined. However, possible triggers include a pathogenic organism (unidentified yet), an immune response to an intraluminal antigen (e.g., cow’s milk protein), or an autoimmune process with immune response to an intraluminal antigen and a similar antigen present on intestinal epithelial cells. In any case, activation of the immune system leads to inflammation of the intestinal tract, both acute and chronic [12–19].
The pathophysiology of IBD is still incompletely understood and is under active investigation, but the common end pathway is inflammation of the mucosal lining of the intestinal tract, causing ulceration, edema, bleeding, and fluid and electrolyte loss. The inflammation of the intestinal mucosa includes both acute inflammation with neutrophilic infiltration and chronic with mononuclear cell infiltration (lymphocytic and histiocytic) [20].
In UC, inflammation always begins in the rectum, extends proximally a certain distance, and then abruptly stops. A clear demarcation exists between involved and uninvolved mucosa. The rectum is always involved in UC, and no “skip areas” are present. UC primarily involves the mucosa and the submucosa, with formation of crypt abscesses and mucosal ulceration. The mucosa typically appears granular and friable. In more severe cases, pseudopolyps form, consisting of areas of hyperplastic growth with swollen mucosa surrounded by inflamed mucosa with shallow ulcers. In severe UC, inflammation and necrosis can in rare cases extend below the lamina propria to involve the submucosa and the circular and longitudinal muscles.
UC remains confined to the rectum in approximately 25 % of cases. In the remainder of cases, UC spreads proximally and contiguously. Pancolitis occurs in 10 % of patients. The small intestine is essentially not involved, except when the distal terminal ileum is inflamed in a superficial manner, referred to as backwash ileitis. Even with less than total colonic involvement, the disease is strikingly and uniformly continuous. As the disease becomes chronic, the colon becomes a rigid foreshortened tube that lacks its usual haustral markings, leading to the lead pipe appearance observed on barium enema. The skip areas (normal areas of the bowel interspersed with diseased areas) observed in CD of the colon do not occur in UC.
CD, on the other hand, consists of segmental involvement by a nonspecific granulomatous inflammatory process. The most important pathological feature is the involvement of all layers of the bowel, not just the mucosa and the submucosa, as is characteristic of UC.
Furthermore, CD is discontinuous, with skip areas interspersed between one or more involved areas. Late in the disease, the mucosa develops a cobblestone appearance, which results from deep longitudinal ulcerations interlaced with intervening normal mucosa. The three major patterns of involvement in CD are (1) disease in the ileum and cecum, occurring in 40 % of patients; (2) disease confined to the small intestine, occurring in 30 % of patients; and (3) disease confined to the colon, occurring in 25 % of patients. Rectal sparing is a typical but not constant feature of CD. However, anorectal complications (e.g., fistulas, abscesses) are common. Much less commonly, CD involves the more proximal parts of the GI tract, including the mouth, tongue, esophagus, stomach, and duodenum. CD causes three patterns of involvement: (1) inflammatory disease, (2) strictures, and (3) fistulas.
The incidence of gallstones and kidney stones is increased in CD because of malabsorption of fat and bile salts. Gallstones are formed because of increased cholesterol concentration in the bile, caused by a reduced bile salt pool. Patients who have CD with ileal disease or resection also are likely to form calcium oxalate kidney stones. With the fat malabsorption, unabsorbed long-chain fatty acids bind calcium in the lumen. Oxalate in the lumen normally is bound to calcium. Calcium oxalate is poorly soluble and poorly absorbed; however, if calcium is bound to malabsorbed fatty acids, oxalate combines with sodium to form sodium oxalate, which is soluble and is absorbed in the colon (enteric hyperoxaluria). The development of calcium oxalate stones in CD requires an intact colon to absorb oxalate. Patients with ileostomies do not develop calcium oxalate stones. Extraintestinal manifestations of IBD include iritis, episcleritis, arthritis, and skin involvement, as well as pericholangitis and sclerosing cholangitis.
The most common causes of death in IBD are peritonitis with sepsis, malignancy, thromboembolic disease, and complications of surgery. Malnutrition and chronic anemia are observed in long-standing CD. Children with CD or UC can exhibit growth retardation.
Patients with UC most commonly present with bloody diarrhea, whereas patients with CD usually present with non-bloody diarrhea. Abdominal pain and cramping, fever, and weight loss occur in more severe cases. The presentation of CD is generally more insidious than that of UC. UC and CD are generally diagnosed using clinical, endoscopic, and histologic criteria. However, no single finding is absolutely diagnostic for one disease or the other. Furthermore, approximately 20 % of patients have a clinical picture that falls between CD and UC; they are said to have indeterminate colitis. Accordingly, imaging may be needed for the detection and for evaluation of the disease activity during its course.
4.4.2 Chest Inflammation
The chest is a common site of various types of infection, acute and chronic. Such infections are frequent in the elderly and in immunosuppressed patients, including cancer patients. Common inflammatory conditions relevant to nuclear medicine include pneumonia, sarcoidosis, diffuse interstitial fibrosis, and Pneumocystis (jiroveci) carinii pneumonia (See also Chap. 12).
4.4.2.1 Sarcoidosis
Sarcoidosis is an inflammatory condition of uncertain etiology characterized by the presence of noncaseating granulomas involving multiple organs. The disease is now recognized as a member of a large family of granulomatous disorders and has been reported from all parts of the world. Current evidence points to genetic predisposition and exposure to yet unknown transmissible agent(s) and/or environmental factors as etiological agents [21]. The lung is most commonly and usually the first site of involvement, and the inflammatory processes extend through the lymphatics to the hilar and mediastinal nodes [22]. The lung is involved in more than 90 % of cases. Pulmonary sarcoidosis starts as diffuse interstitial alveolitis, followed by the characteristic granulomas. Granulomas are present in the alveolar septa as well as in the walls of the bronchi and pulmonary arteries and veins. The center of the granuloma contains epithelioid cells derived from mononuclear phagocytes, multinucleated giant cells, and macrophages. Lymphocytes, macrophages, monocytes, and fibroblasts are present at the periphery of the granuloma [23]. Sarcoidosis represents a challenge to clinical investigation because of its unpredictable course, uncertain response to therapy, and diversity of potential organ involvement and clinical presentations [24]. The diagnosis is based on a compatible clinical and/or radiological picture, histopathological evidence of noncaseating granulomas in tissue biopsy specimens, and exclusion of other diseases capable of producing similar clinical or histopathological appearances. Even microscopically, the noncaseating granulomas are not specific [21]. Infection by mycobacterial species other than Mycobacterium tuberculosis frequently leads to the production of noncaseating granulomas [25]. The condition is underdiagnosed in some areas. However, owing to the increasing awareness, it is being diagnosed more frequently than a few decades ago [26].
The disease runs a benign course with spontaneous remission of the activity though some degree of residual pulmonary function abnormality persists. Only a minority of patients develop complicated disease that may lead to blindness, renal failure, liver failure, and heart involvement.
Corticosteroids remain the mainstay of treatment. Treatment under close clinical monitoring should be tailored to suit the needs of the individual patient hence the need to evaluate disease activity [26].
Advanced age, the presence of pulmonary symptoms, the presence of parenchymal lesions on chest radiograph, a previous history of treatment with corticosteroids, and the presence of extrathoracic involvements at the time of detection are possible prognostic factors in patients with sarcoidosis [27]. The mode of onset and the extent of the disease are also related to prognosis. An acute onset with erythema nodosum or asymptomatic bilateral hilar lymphadenopathy usually heralds a self-limiting course, whereas an insidious onset, especially with multiple extrathoracic lesions, may be followed by relentless, progressive fibrosis of the lungs and other organs
4.4.2.2 Pneumocystis carinii (jiroveci) Pneumonia
Pneumocystis carinii (jiroveci) pneumonia (PCP) is a condition that may be endemic or epidemic. It is caused by Pneumocystis carinii, which was considered as a protozoon and recently as a fungus. The condition is common in premature infants, debilitated children, and in other immunocompromised conditions, particularly the acquired immune deficiency syndrome (AIDS), but it is also seen in congenital immunodeficiency and in patients who are receiving chemotherapy and corticosteroids. It remains a significant cause of morbidity and mortality in human immunodeficiency virus and nonhuman immunodeficiency virus-associated immunosuppressed patients [28]. It is the most common infection in AIDS patients, and it remains an important cause of morbidity and mortality [29]. The introduction of highly active antiretroviral therapy in industrialized nations however has led to dramatic declines in the incidence of AIDS-associated complications, including PCP. In the developing countries, no decline has occurred [30]. Transmission is usually airborne. The pathological changes are predominantly in the lungs with an inflammatory reaction consisting of plasma cells of variable amount, monocytes, and histiocytes. This disease has also been reported in immunocompetent patients, and in this case the presentation is more closely resembling the disease of immunocompromised patients other than AIDS patients [31, 32]. The diagnosis is currently established through identification of the organisms in bronchial secretions obtained by bronchoalveolar lavage or bronchial washings [33]. Gallium-67 is an important imaging modality that helps in the diagnosis and evaluation of the activity of the disease.
4.4.2.3 Interstitial Pulmonary Fibrosis
Interstitial pulmonary fibrosis, a sometimes fatal condition, is characterized by parenchymal inflammation and interstitial fibrosis. The pathological changes start with alveolitis; this is followed by derangement of the alveolar-capillary units, leading to the end stage of fibrosis. There is a correlation between the inflammatory activity and the amount of gallium-67 activity in the lungs [34].
4.4.3 Renal Inflammation
Urinary tract infection (UTI) is common particularly in children. There are two main varieties of acute renal infection: pyelitis, which is confined to the renal pelvis, and pyelonephritis, where the renal parenchyma is also involved. It is not always possible to differentiate between the two conditions on clinical grounds. The pathology of acute pyelitis is not very well understood. The importance of the condition, however, lies in the fact that recurrent subclinical attacks are believed to be significant in the pathogenesis of chronic pyelonephritis [35].
The number of patients with chronic kidney disease and consequent end-stage renal disease is rising worldwide [36]. End-stage kidney disease, defined as that requiring dialysis or receipt of a transplant or that which may lead to death from chronic kidney failure, generally affects less than 1 % of the population [37]. Among today’s challenges is to identify those at greatest risk for end-stage renal disease and intervene effectively to prevent progression of early chronic kidney disease and conditions leading to chronic disease [37].
Rarely, uncomplicated acute pyelonephritis causes suppuration and renal scarring. However, urinary infections in patients with renal calculi, obstructed urinary tract, neurogenic bladder, or diabetes are frequently much more destructive and have ongoing sequelae [38].
4.4.3.1 Acute Pyelonephritis
Acute pyelonephritis is a common medical problem. The diagnosis and management of this condition is complex. Patients initially diagnosed with pyelonephritis typically exhibit symptoms and laboratory evidence suggesting infected urine, with signs referable to upper urinary tract infection. However, no consistent set of signs and symptoms are sensitive and specific for this diagnosis. Symptoms of acute pyelonephritis generally develop rapidly over a few hours or a day. Symptoms of lower UTI may or may not be present. These include dysuria; urinary frequency, hesitancy, and urgency; gross hematuria; and suprapubic discomfort, heaviness, pain, or pressure. Additionally, flank pain and tenderness, unilateral or sometimes bilateral, are present. Fever is not always present. When present, it is not unusual for the temperature to exceed 103 °F (39.4 °C). Rigor, chills, malaise, and weakness may be present. Anorexia, nausea, vomiting, and diarrhea may also be present. Most patients have significant leukocytosis, pyuria with leukocyte casts in the urine, and bacteria on a gram stain of unspun urine.
Many conditions and clinical situations are associated with an increased risk of pyelonephritis. Table 4.2 lists common risk factors.
Table 4.2
Common risk factors for pyelonephritis
Mechanical factors |
Obstruction |
Prostatic infection |
Calculi |
Urinary diversion procedure |
Infected cysts |
External drainage with urinary catheters or nephrostomy tubes |
Stents |
Vesicoureteral reflux |
Neurogenic bladder |
Bladder or renal abscesses |
Fistulas |
Recent urinary tract instrumentation |
Metabolic and hormonal factors |
Diabetes mellitus |
Pregnancy |
Renal impairment |
Malakoplakia |
Primary biliary cirrhosis |
Immune factors |
Transplant recipients |
Neutropenia |
Congenital or acquired immunodeficiency syndromes |
Infectious factors (unusual pathogens) |
Yeasts and fungi |
Mycoplasma species |
Resistant bacteria, including P. aeruginosa |
Calculi-predisposing bacteria, including Proteus species and Corynebacterium urealyticum |
Other factors |
Uncircumcised penis |
Old age |
Recent antimicrobial use |
Pyelonephritis is significantly more common in females (higher in white than in black) compared to males. Approximately 10–30 % of women develop a symptomatic UTI at some point in their lives.
Acute pyelonephritis is a bacterial infection of the kidney with acute inflammation of the pyelocaliceal lining and renal parenchyma centrifugally along medullary rays. This can occur by more than one way. Most often it occurs because of ascending infection from the lower urinary tract (Fig. 4.4). The initial colonization of the walls of the ureter is in areas of turbulent flow which leads to paralysis of peristalsis. Dilation and functional obstruction result in subsequent pyelonephritis. Another way is by direct reflux of bacteria. Hematogenous spread to the kidney by gram-positive and less likely by gram-negative organisms is the third way that can occur. This has become less prevalent since the advent of rapid use of antibiotics. Little or no evidence supports lymphatic spread.
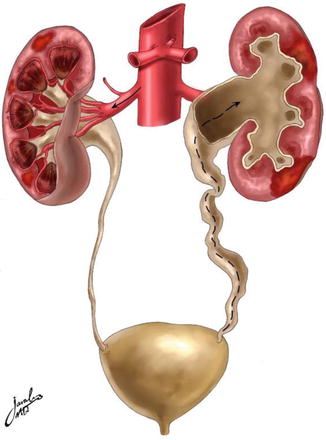

Fig. 4.4
Diagram illustrating the routes of inducing urinary tract infection. The left-hand side represents the hematogenous route, while the right-hand side represents the retrograde route such as with vesicoureteral reflux
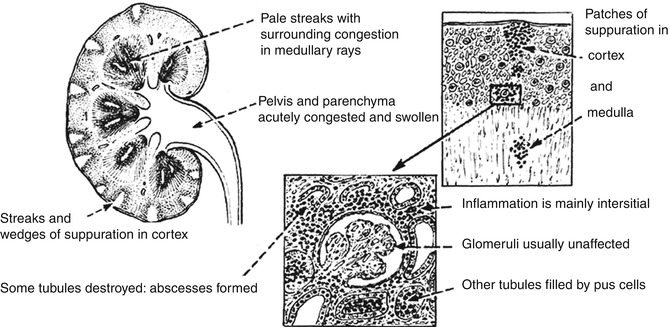
Grossly, the kidney is enlarged and edematous. The cut surface may show small abscesses in the cortex, and more often there are wedge-shaped purulent areas streaking upward from the medulla, with normal areas of the kidney tissue intervening in between infected zones (Fig. 4.5). Frequently, the pelvis and calyces are inflamed and dilated. In severe infection, renal papillary necrosis may be present.
Microscopically, there is intense inflammation, with infiltration of polymorphonuclear leukocytes throughout the interstitial tissue and abscess formation. There is destruction of the tubules, but the glomeruli and blood vessels are often unaffected. The disease remains essentially focal in character, with areas of normal tissue. Following treatment and removal of predisposing factors such as obstruction, healing may occur, leaving coarse scars which stretch from the medulla to the capsule of the kidney.
4.4.3.2 Chronic Pyelonephritis
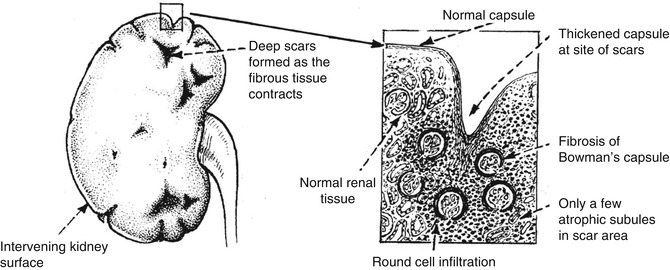
Chronic pyelonephritis is a chronic condition affecting the pelvis and parenchyma and resulting from recurrent or persistent renal infection. It occurs almost exclusively in patients with major anatomic anomalies, including urinary tract obstruction, struvite calculi, renal dysplasia, or, most commonly, vesicoureteral reflux (VUR) in young children. Grossly, the kidney shows normal areas alternating with zones of scarring. Wedge-shaped scars can be seen on the subcapsular surface of the kidney. The appearance differs, depending on the presence or absence of obstruction. Chronic pyelonephritis in the presence of intra- or extrarenal obstruction shows dilation of the pelvocalyceal system and sometimes peripelvic fibrosis. If no obstruction is present, the pelvic change is in the form of peripelvic fibrosis rather than dilation (Fig. 4.6).
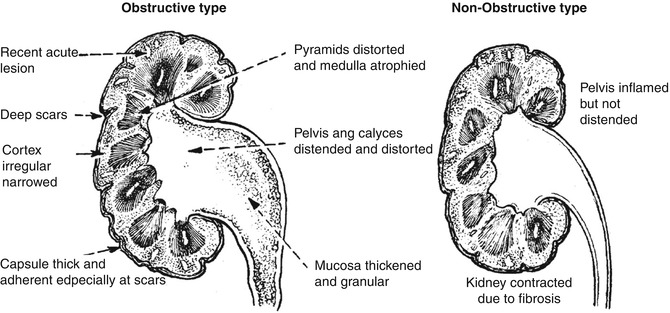

Fig. 4.6
Types of pyelonephritic changes based on whether obstruction is present (From [41] with permission)
Microscopically, the scarred areas show changes in the interstitium and tubules. The interstitial tissue shows infiltration by predominantly lymphocytes and plasma cells. The tubules become atrophic and may collapse (Fig. 4.7). The glomeruli may be normal in some cases, while in others periglomerular fibrosis is present.
4.5 Pathophysiology of Major Skeletal Inflammations
Osteomyelitis indicates an infection involving the cortical bone as well as the marrow (see Chap. 6). It is classified into many types based on several pathological and clinical factors [42–49] including route of infection, patient age, etiology, and onset. Hematogenous osteomyelitis most commonly affects children, and the metaphyses of long bones are the most common sites. Nonhematogenous osteomyelitis, on the other hand, occurs as a result of penetrating trauma, spread of a contiguous soft tissue infection, or inoculation, as in drug addicts [48–54]. Many organisms have been encountered in the pathogenesis of osteomyelitis, particularly gram-positive organisms, the most common being Staphylococcus aureus [44–46]. Like many other pathological conditions of bone, infections cause reactive new bone formation which – among other factors, particularly increased blood flow – is the principle reason for the accumulation of bone-seeking radiopharmaceuticals at the site of skeletal infections.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree