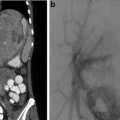
Fig. 5.1
FMD in a 12-year-old with hypertension. Angiographic findings typical for the intimal fibroplasia type of FMD

Fig. 5.2
FMD in a 4-year-old with hypertension. Angiographic findings reveal long-segment smooth stenosis with post-stenotic dilation of an intrarenal segmental artery
Neurofibromatosis I (Figs. 5.3–5.5)




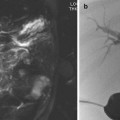
Fig. 5.3
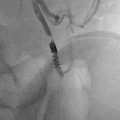
NF1 and severe renal artery stenosis. (a) Angiogram of left kidney reveals irregular main renal artery with stenosis. (b) Post angioplasty reveals slight improvement but considerable narrowing and irregularity persists. Patient had some improvement but developed restenosis 2 years later requiring repeat angioplasty

Fig. 5.4
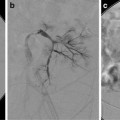
NF1 and hypertension. Angiogram of the left kidney reveals multiple aneurysms in the renal hilum. No endovascular options were considered in this patient

Fig. 5.5
NF1 with difficult balloon dilation. Angiogram reveals irregularity and narrowing of the main renal artery. Balloon waist persists during dilation
Midaortic syndrome (Fig. 5.6)


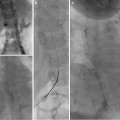
Fig. 5.6
Midaortic syndrome with bilateral renal artery stenosis. (a) Angiogram reveals narrowing of the aorta and proximal renal arteries. (b, c) Brachial access used for bilateral angioplasties
William’s syndrome
Takayasu’s arteritis (Fig. 5.7)


Fig. 5.7
Takayasu’s arteritis. Focal narrowing and ectasia of the main and segmental renal arteries are seen in this patient found to have large vessel abnormalities throughout the upper extremities and thoracic vessels as well
Polyarteritis nodosa
Radiation therapy
Trauma (Fig. 5.8)


Fig. 5.8
Traumatic injury of the renal artery from motor vehicle accident. (a, b, c) CTA reveals poor perfusion of the right kidney, and reconstructions show a mid-renal artery lesion. (d) Selective angiography shows an intimal dissection of the mid-renal artery. (e) Angioplasty was successful in revascularizing the kidney
Congenital rubella syndrome
Tuberous sclerosis
Marfan’s syndrome
Klippel-Trenaunay-Weber
Epidermal nevus syndrome
Tumor encasement (neuroblastoma)
Technique
Preprocedure
Hypertensive medications are generally not withheld even if treatment is to be undertaken. Renal function should be known with BUN and creatinine levels checked within last 30 days.
Anesthesia
General anesthesia is recommended for all diagnostic and interventional procedures. General anesthesia can provide apnea eliminating respiratory motion and also provides for longer procedures.
Access
The femoral artery is most often used for access and is always using ultrasound guidance with a 4.5 Fr vascular sheath inserted. We have on occasion used brachial artery or even carotid artery access (with assistance of cardiovascular surgery) for patients in whom unfavorable renal artery or aorta anatomy makes cephalad access preferable.
Anticoagulation
Heparin, 50–100 units/kg, is given after insertion of the vascular sheath.
Aortogram
Aortogram is almost always performed first. This provides an assessment of the main renal arteries prior to any guidewire or catheter instrumentation of the renal artery that may produce spasm and mimic a stenosis (Fig. 5.9). A 4 Fr pigtail catheter is preferred and positioned below the superior mesenteric artery to avoid reflux into overlying arteries. Renal arteries usually arise at the level of L1–L2.


Fig. 5.9
Selective arteriogram of right kidney reveals an area of spasm in the lower pole branch not present on the aortogram. (b) During selective catheterization the guide wire advanced into the lower pole artery causing spasm
Contrast volume: As a general rule, the volume necessary for an upper abdominal aortogram is 1 cm3/kg. Below the celiac artery and SMA, 0.8 cm3/kg up to an adult volume of 25 cm3 should be adequate.
Flow rate should be such that entire volume is injected over 1–1.5 s. Most 4 Fr pigtail catheters have a limit of 17 cm3/s maximum rate at 1,200 psi.
Selective Arteriography
Every renal arteriogram should have selective catheterizations to evaluate for small lesions that may be missed on the aortogram (Fig. 5.10). A 4 Fr reverse curve (Sos 1 or 2) catheter is preferred to catheterize each renal artery. Cobra-shaped catheters will often recoil out of the renal artery; thus, the reverse curve shapes are preferred. Thirty to forty percentage of patients will have multiple renal arteries and each should be selectively catheterized.


Fig. 5.10
Segmental stenosis of right lower pole branch seen on selective angiogram. This lesion was not apparent on the aortogram performed with a flush catheter from the abdominal aorta. (a) lower pole stenosis demonstrated. (b) Following angioplasty. (c) improved lower pole blood flow was seen
Hand injections are often performed in small children with volumes of 3–5 cm3. Large adolescent children will require adult rates of 6–8 cm3/s and volumes of 10–15 cm3.
DSA imaging is required with apnea. Rapid sequences of 4 frames/s for the aortogram are desired. Three frames/s during the selective angiograms are used. Two oblique views are obtained with approximately 5 and 25° of angulation toward the ipsilateral side.
The diagnostic arteriogram should be undertaken with the most careful technique so that isolated small intrarenal lesions are not overlooked.
Angiographic Findings
There can be a number of direct and indirect findings indicating the presence of a significant vascular lesion:
Stenosis
Stenosis may be seen secondary to a number of causes listed above. The most common etiology is fibromuscular disease (FMD) which is an idiopathic angiopathy characterized by noninflammatory fibrodysplastic narrowing of medium-size arteries. FMD has been classified into intimal, medial, and perimedial subtypes. Lesions with intimal fibroplasia generally demonstrate highly stenotic localized lesions often with post-stenotic dilation. Perimedial fibroplasia is characterized by severe focal stenoses and mild beading. Medial fibroplasia is characterized by more typical beaded appearance. Approximately 50 % of children with renal artery stenosis will have main renal artery disease, and the other half may have isolated intrarenal stenoses. This underscores the need for careful evaluation of the selective renal artery injections (Fig. 5.11).


Fig. 5.11
FMD in a 5-year-old patient with hypertension. (a) Selective angiography reveals a relatively focal narrowing of the upper pole main renal artery. (b) Post angioplasty (4-mm balloon) with guidewire still across the area of dilation, an angiogram reveals the improved angiographic results
Post-stenotic Dilation
Post-stenotic dilation may be the only abnormality seen and should prompt additional views be obtained to try and identify a subtle stenosis or web (Figs. 5.12 and 5.13).



Fig. 5.12
A 6-year-old with hypertension. (a) Selective angiogram reveals a suspicious “dilated” vessel in the mid-polar region of the left kidney. (b) Following additional oblique projection angiograms, a rotational angiogram with 3D reformations was performed confirming a stenosis with post-stenotic dilation of a small lower pole interlobular artery. (c) Selective catheterization of this vessel was performed with microcatheter and the segmental artery embolized with ethanol (4 mL). Post-embolization angiogram reveals occlusion of the artery. The patient’s hypertension resolved

Fig. 5.13
Upper pole stenosis with post-stenotic dilation. The initial finding was the mildly ectatic (post-stenotic dilation) upper pole vessel. A web stenosis was only seen after several oblique projections were done
Intrarenal Collateral Vessels
Intrarenal collateral vessels are seen in approximately 50 % of patients with stenotic lesions. Identification of collaterals should lead to thorough assessment of entire arterial anatomy as the stenoses may be subtle and readily seen. Careful angiography with additional oblique projections or rotational angiography may be necessary to identify subtle stenoses (Figs. 5.14 and 5.15).


Fig. 5.14
Intrarenal segmental arterial occlusion with collaterals and delayed upper pole artery filling. (a) An early arterial phase angiogram reveals extensive collateral vessels in the mid-portion of the kidney and nonvisualization of normal upper pole arteries. (b) A delayed image reveals the upper pole artery filling solely through the intrarenal collaterals. Angioplasty and embolization options were unsuccessful in this patient