Fig. 13.1
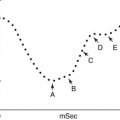
Diagram of the lobes and segments of the lungs
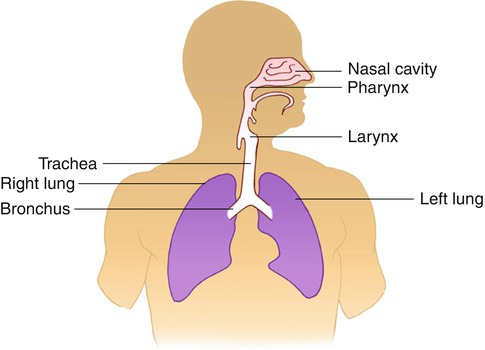
Fig. 13.2
Simple diagram of the upper and lower airways
13.1.1 Respiratory Airways
The upper airways are lined by a ciliated mucosa, richly supplied with blood, which warm and humidify the inspired air and get rid of foreign particles. The air normally flows by way of the nose, nasopharynx, and oropharynx to the lower airways. When the nose is obstructed or additional flow of air is needed, as during exercise, air flows via the mouth and oropharynx to the lower airways. Foreign particle removal and humidification are not efficient with mouth breathing as compared with the usual breathing through the nose.
The lower airways are formed of a conducting system and a gas exchange system (Fig. 13.3). The trachea divides into two main bronchi at the carina, and each bronchus enters the corresponding lung at the hilum along with the pulmonary blood vessels and lymphatic channels. The trachea measures up to 25 cm in length and 2.5 cm in diameter. The right main bronchus extends to the right lung more vertically than the left bronchus to the left lung. This explains the more frequent aspiration of foreign material in the right side. At the hila, the bronchi divide to lobar bronchi, then segmental and subsegmental bronchi, and then into smaller bronchioles, and at the 16th division, the tracheobronchial tree ends in the tiny terminal bronchioles which form the ends of the conducting airways and are followed by the gas exchange airways. The lung segments are individual units with their bronchovascular supply; hence, they can be individually resected. The airways responsible for conducting air from outside the body into the lungs are lined by ciliated mucous membranes. The cilia, which are hairlike projections, act as sweepers to prevent dust and foreign particles from passing distally into the lungs. Damage to the respiratory epithelium and its cilia allows bacteria and viruses to proliferate and induce infection.
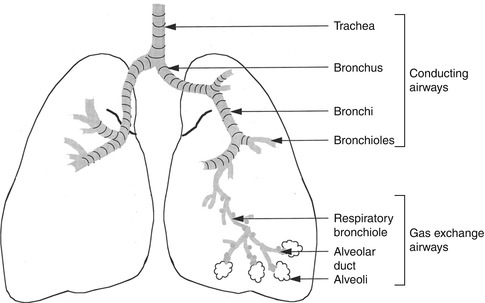

Fig. 13.3
The trachea, bronchi, and bronchioles form the tracheobronchial tree, so called since it resembles an inverted tree. The conducting system is composed of the trachea, bronchi, and bronchioles up to the 16th division and is lined by ciliated mucosa. The gas exchange system consists of the more distal bronchioles (respiratory) and the alveoli that are lined by nonciliated mucus membrane
The gas exchange airways start where the terminal bronchioles divide further into smaller, respiratory bronchioles which include increasing numbers of alveoli as the division progresses. By the 23rd division, the respiratory bronchioles end in alveolar ducts that lead to alveolar sacs which are made up of numerous alveoli. The alveoli are extremely thin-walled sacs surrounded by capillaries and are the primary site of gas exchange. At birth there are approximately 25 million alveoli; this increases to 300 million in adults. The alveoli are lined by type I alveolar cells that provide structure to the alveolar wall and type II cells that secrete a lipoprotein, the surfactant which coats the alveolar inner surface and aids its expansion during inspiration [1].
Ventilation describes the process by which air flows in and out of the gas exchange airways. Ventilation is involuntary most of the time and is controlled by the sympathetic and parasympathetic autonomic nervous systems, which adjust the caliber of the airway via contraction and relaxation of the bronchial smooth muscle and control the depth and rate of ventilation.
The nose and trachea trap most particles of more than 10 μm in diameter, while the cilia of the bronchi and bronchioles pick up particles 2–10 μm in diameter that are deposited in these airways. Smaller particles remain airborne till they are deposited in the alveoli and removed by macrophages. Extremely small particles behave as a gas and are breathed out. This is the basis of scintigraphic ventilation studies using radioactive aerosols and gases. The flow of oxygen through the 99mTc DTPA reservoir should create small aerosol particles to be airborne and deposited distally in the alveoli. Larger particles are deposited in the more proximal airways and influence the quality of ventilation studies. This also explains the longer biologic clearance of aerosols compared with radioactive gases, which are breathed out without deposition.
13.1.2 Pulmonary Vasculature
The lung is supplied by two different blood circulations. The pulmonary circulation is a low-pressure, low-resistance system through which oxygen enters and carbon dioxide is removed. The bronchial circulation is a part of the high-pressure systemic circulation that supplies oxygenated blood to the lung tissue itself.
The pulmonary circulation contains the vast majority of blood present in the lung, and since it has lower pressure than systemic circulation, its vessels have a thinner muscle layer. The mean pulmonary artery pressure is 18 mmHg, compared with 90 mmHg for the aorta. The gas exchange airways are served by this pulmonary circulation, which is considered a separate division of the circulatory system. The pulmonary circulation is carried through the pulmonary artery, which branches out to two main pulmonary arteries, one to each lung, entering at the hilum. It then divides progressively into smaller branches, following the branches of the bronchial tree to the smallest, precapillary arterioles, which divide to form a capillary network surrounding the alveoli. The membrane that surrounds the alveoli and contains the capillaries is called the alveolocapillary membrane [2].
The precapillary arterioles are approximately 35 μm in diameter and number approximately 300 million in adults. The capillaries, 7–10 μm in diameter, number 300 billion in adults. The more proximal terminal arterioles have a diameter of approximately 100 μm. This basic anatomical fact is important in determining the size of particles injected for perfusion studies; they should be less than 100 μm to prevent blocking of the terminal arterioles [3].
Although the pulmonary circulation is innervated by the autonomic nervous system, vasodilation and vasoconstriction are controlled mainly by local and humoral factors, particularly arterial oxygenation and acid–base balance. Vasoconstriction of the pulmonary arterial system occurs secondary to alveolar hypoxia and acidemia and by the presence of inflammatory mediators such as histamine, bradykinin, serotonin, and prostaglandin.
The bronchial circulation, on the other hand, carries approximately 5 % of the blood coming to the lungs and is part of the systemic circulation. In contrast to the pulmonary circulation, it does not participate in gas exchange. It supplies the tracheobronchial tree, large pulmonary vessels, and other structures of the lungs, including the pleurae, with blood.
13.1.3 Respiratory Function
The major function of the respiratory system is to oxygenate the blood and remove waste products of the body in the form of carbon dioxide. Oxygen in the inhaled air diffuses from the alveoli into the surrounding blood in the capillaries, where it attaches to hemoglobin molecules and red blood cells and is carried to the various tissues of the body. Carbon dioxide, on the other hand, as a waste product of cellular metabolism, diffuses in the opposite direction, from the blood in capillaries into the alveoli, and is removed from the body during expiration.
The respiration is controlled by the respiratory center in the medulla at the base of the brain. The respiratory center in the brain stem sends impulses to respiratory muscles to contract and relax. The respiratory center also receives impulses from two main types of peripheral receptors, neuro- and chemoreceptors. Neuroreceptors (lung receptors) monitor the mechanical aspects of ventilation such as the need to expel unwanted substances and expansion of the lungs. The chemoreceptors in the brain circulatory system monitor the pH status of the cerebrospinal fluid and arterial oxygen content (PO2) to regulate ventilation accordingly.
Any change in the carbon dioxide in the blood will affect the rate and depth of respiration. A slight increase in carbon dioxide concentration in the blood increases the rate and depth of respiration, such as when the individual exercises, since the accumulated waste gas must be removed from the body.
This increase in respiratory rate and depth is secondary to the stimulation of the muscles of respiration, which include the diaphragm and the intercostal muscles, by the respiratory center. Contraction of these muscles causes the volume of the chest cavity to increase, with a consequent drop in the pressure within the lungs, and forces air to move into the tracheobronchial tree. When these respiratory muscles relax, the volume of the chest cavity decreases, the pressure increases, and the air is pushed out of the lungs. When breathing is difficult, or in patients with obstructive airway disease, special muscles of expiration, abdominal and internal intercostal muscles, may be additionally needed.
13.1.4 Distribution of Ventilation and Perfusion
Normally, the lower zones of the lungs are better perfused and ventilated because of the effect of gravity. This gradient is more pronounced in perfusion than in ventilation (Fig. 13.4). This physiological fact will usually cause the perfusion to appear less than the ventilation in the lung apices on scintigraphy. This should not be confused with a mismatching pattern. 99mTc-macroaggregated albumin (MAA) is injected for perfusion imaging while the patient is in the supine position to minimize the gradient. Injection while the patient is taking a deep breath also helps.
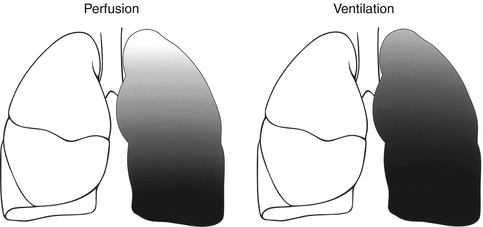

Fig. 13.4
The gradient pattern in perfusion and ventilation of the lungs (From Elgazzar et al. [23] with permission)
13.2 Pulmonary Embolic Disease
Pulmonary embolism is potentially fatal and the most common pathological condition involving the lungs of hospitalized patients. The majority of fatal emboli are not recognized or suspected prior to death.
13.2.1 Pathogenesis and Risk Factors
The vast majority of pulmonary emboli are thromboemboli originating from deep veins. Fat, air, or tumor emboli are rare [4]. Fat emboli are reported with long bone fractures and liposuction, while air emboli occur in cardiac and neurosurgeries. Renal cell carcinoma with invasion reaching inferior vena cava is a clinical setting that may lead to tumor emboli. Data indicate that 90 % of pulmonary thromboemboli originate from the lower extremities and pelvis. The remainder comes from thrombi that occur in the right side of the heart or in bronchial or cervical veins. Embolization and symptomatology are proportional to how proximal is the vein that contains the thrombus. The vast majority of pulmonary thromboemboli originating from thrombi of the lower extremities come more frequently from the thigh and pelvis (75 %) than from smaller veins of the calf and feet [5, 6]. Septic embolus refers to an infected thromboembolus which occurs either on site or secondary to detachment of an infected vein thrombus of the lower extremities. The risk of pulmonary embolus is also directly related to the presence of a residual clot at the site of a venous thrombus [7].
13.2.2 Deep Venous Thrombosis
The best solution to the problem of embolism is to prevent it. However, prevention requires identification of those at risk. Perhaps the most important step in defining who is at risk for this disorder has been the recognition that pulmonary emboli arise from the sites of deep venous thrombosis, almost exclusively in the lower extremity veins. Therefore, those at risk for deep venous thrombosis are those at risk for pulmonary embolism. The classical risk triad elucidated by Virchow in the nineteenth century includes venous stasis, intimal injury, and alteration in coagulation. These are the primary factors in the pathogenesis of venous thrombosis. Deficiencies of antithrombin III, protein C, protein S, and protein Z are clearly important, as is the presence of lupus anticoagulant. There are other rarer conditions such as homocystinuria and deficiencies of the fibrinolytic system. More factors are being identified, but at the present time, up to 90 % of all patients with thromboembolism have no identifiable coagulopathy. Thus, in most patients, some clinical states associated with venous stasis, intimal injury, or both are the basis for an increased risk of deep venous thrombosis. These clinical states include injury to the pelvis or lower extremities, surgery involving the lower extremities, all surgical procedures requiring prolonged (at least 30 min) general anesthesia, burns, pregnancy and the postpartum state, previous venous thrombosis with residual obstruction, right ventricular failure of any cause, occupations in which prolonged venous stasis is involved, and any cause of immobility. Other risk factors are age (particularly above 70 years), obesity, cancer and the use of estrogen-containing medications, neoplasm, infection in the immediate area of veins, and hypercoagulability (Table 13.1).
Table 13.1
Risk factors for deep vein thrombosis and pulmonary thromboembolism
1. Postoperative state especially following operations on the abdomen and pelvis |
2. Trauma, including fractures, particularly of the lower extremities |
3. Neoplasms |
4. Prior history of thromboembolic disease |
5. Venous stasis |
6. Vascular spasm |
7. Intimal injury |
8. Hypercoagulability states |
9. Immobilization |
10. Infection of the area in the immediate vicinity of veins |
11. Heart disease, especially: |
Myocardial infarction |
Atrial fibrillation |
Cardiomyopathy |
Congestive heart failure |
12. Pregnancy |
13. Polycythemia |
14. Hemorrhage |
15. Obesity |
16. Old age |
17. Varicose veins |
18. Certain drugs such as oral contraceptives, estrogens |
19. Following cerebrovascular accidents |
An important point to note is that risk factors should be regarded as cumulative, not independent. These factors allow the establishment of a “risk profile” for a given patient, a profile that conditions the intensity of prophylactic initiatives. The anatomical location of the deep venous thrombosis affects as well the likelihood of extending into a pulmonary embolism as noted earlier.
Venous thrombi appear to begin either in the vicinity of a venous valve, where eddy current arises, or at the site of intimal injury. Platelet aggregation and release of mediators initiate the sequence. With stasis, there is local accumulation of coagulation factors; the coagulation cascade is activated, and the characteristic red fibrin thrombus develops. Pathologically there will be a platelet nidus from which a large fibrin thrombus extends.
Regarding the natural history, one of three events can happen after the formation of the thrombus. First, the red thrombus grows explosively and obstructs the vein completely. This can happen even within a few minutes. Second, partial venous obstruction may occur. Blood flow therefore continues over the thrombus surface. Under this circumstance, thrombus growth tends to occur by the progressive layering of platelets and fibrin on the clot surface, pathologically seen as the lines of Zahn. Third, probably the most common scenario, a small thrombus is swept away before it reaches an appreciable size. It lodges in the pulmonary vasculature without symptoms.
Unless fibrinolytic resolution is prompt, organization of the thrombus begins within hours of formation. The thrombus is slowly replaced by granulation tissue. This process anchors the thrombus to the venous wall.
The dynamic battle between fibrinolysis and thrombus formation is fought out over a period of 7–10 days, at the end of which time either complete resolution has occurred or an endothelialized residual is present. At any time during this period, a portion or all of the thrombus can detach as an embolus. This risk is highest early, before significant dissolution or organization occur [6].
13.2.3 Pulmonary Thromboembolism
13.2.3.1 Consequences
Pulmonary thromboemboli occur more commonly in the lower lobes because of the preferential blood flow to these regions. This also applies to the right lung because of the straighter course of the pulmonary artery. Immediately after acute embolism, there is a decrease of perfusion distal to the occluded vessel along with a transient decrease of ventilation to the affected segment. The blood flow is diverted to the other portions of the lung, and pulmonary artery pressure may increase, although cardiac output usually remains stable. The resultant tissue ischemia disturbs certain metabolic functions of the lung such as the production of surfactant. Reduction of the surfactant concentration reduces the alveolar surface tension and may cause the atelectasis that often accompanies embolism. If the embolus completely occludes an artery or an arteriole and the collateral bronchial circulation is insufficient to sustain tissue viability, infarction occurs over 24–48 h. Pulmonary infarction with coagulative necrosis results in an area of radiographic opacity that requires an average of 20 days to resolve but occurs in less than 10–15 % of patients with pulmonary embolism. There is significant inflammatory component in pulmonary infarcts which is the basis behind the reported significant FDG uptake in recent lung infarcts and can cause false-positive interpretation for lung malignancy [8]. More frequently, incomplete infarction with hemorrhage but without necrosis occurs. This type of injury resolves quickly and produces only transient radiographic opacities. Infarction always involves the pleural surface of the lung (peripheral) and more frequently involves the lower lobes than other sites.
The regional decrease in ventilation is due to local bronchoconstriction with a tendency for redistribution of ventilation away from the hypoperfused segment. This probably occurs due to decreased regional alveolar and airway carbon dioxide tension, which is the usual stimulus for bronchodilation. This hypocapnia is corrected quickly, since patients inhale carbon dioxide-rich tracheal “dead space air” into the alveolar zones after the embolic event, raising the alveolar pCO2 [6]. The release of neurohumoral factors, most importantly serotonin and thromboxane A2, also causes bronchoconstriction. These factors are released after embolization by activated platelets and mediate bronchospasm of small airways through their effects on the smooth muscles [9]. The ventilation of the hypoperfused areas returns to normal within several hours after acute embolism [10, 11]. This concept is the pathophysiological basis for the scintigraphic interpretation of ventilation and perfusion scans, which show segmental perfusion defects with preserved ventilation as a typical scintigraphic pattern for pulmonary embolism. Those showing only regions of matched perfusion and ventilation defects carry a low probability of pulmonary embolism if no chest X-ray abnormalities are noted at the same sites, since this pattern is more likely associated with nonembolic conditions and is more typical of parenchymal lung disease. Because patients with pulmonary emboli usually arrive at the hospital after normalization of the ventilation at the site of pulmonary emboli, the mismatching pattern is typical of pulmonary emboli. However inpatients may have their V/Q scans within a short time after presentation and matching abnormalities may be associated with pulmonary emboli. This has to be borne in mind, and the duration of symptoms should be a factor in decision-making regarding the management of pulmonary embolism.
Some degree of arterial hypoxemia may also occur, one reason being the widening of the arteriovenous oxygen difference caused by acute right ventricular failure. Another reason is the enhanced perfusion of poorly ventilated or nonventilated lung zones. Loss of pulmonary surfactant may add to the hypoxemia. Hyperventilation almost always occurs and may partly explain the normal levels of oxygen arterial pressure seen in 10–25 % of patients with pulmonary emboli.
An increase in the resistance of the pulmonary arterial circulation, due primarily to mechanical blockage by numerous small emboli in the pulmonary vasculature and also to humorally mediated vasoconstriction, may follow pulmonary emboli. These hemodynamic consequences may include increased pulmonary arterial resistance with elevated pulmonary arterial and right ventricular systolic pressures and hypoxemia. When pulmonary hypertension occurs, it indicates at least 25 % obstruction of pulmonary vascular tree as assessed by angiography [12]. The higher the degree of obstruction, the more severe the abnormalities of the cardiopulmonary hemodynamics become. When over 50 % of the pulmonary vasculature is included (massive pulmonary embolism), acute pulmonary hypertension and/or right ventricular failure (cor pulmonale) occurs [12]. Systemic hypoxemia results from pulmonary arteriovenous shunting and from perfusion of hypoventilated lung segments (V-P imbalances). The AV shunting accounts for the clinical observation that administration of 100 % oxygen will only partially correct hypoxemia induced by pulmonary emboli [11].
The physiological consequences of pulmonary embolism depend on the size of the embolic mass and the general status of the pulmonary circulation. In young individuals with good cardiovascular function and good collateral circulation, thrombi of a large central vessel may be associated with only minimal functional impairment if any. On the other hand, in patients with cardiovascular or severely debilitating diseases, pulmonary embolism may lead to infarction.
13.2.3.2 Resolution
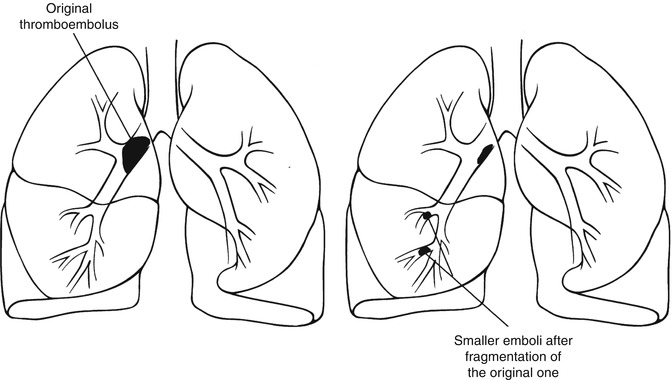
Pulmonary emboli may, spontaneously or with treatment, fragment into smaller portions that travel distally and block smaller arterioles (Fig. 13.5). This may create new, smaller perfusion defects that are more peripherally located in comparison to the original defect caused by the original embolus. This pattern should not be mistaken for recurrent pulmonary emboli on a follow-up scan. If this pattern is the only interval change with no other defects seen in areas other than those in the vicinity of the distribution of the original embolus, it does not suggest recurrent emboli [11].
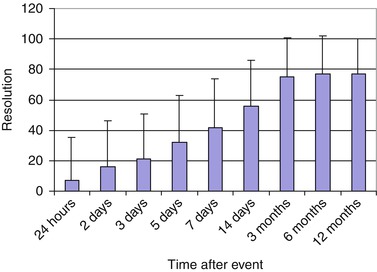
Resolution of pulmonary thromboembolus may start within hours. It can be seen on perfusion scans as early as 24 h and is progressively noted up to 3 months, with insignificant change after 6 months (Fig. 13.6). This is the basis of the recommendation that follow-up ventilation and perfusion scans are performed 3 months after the initial incident for evaluation of resolution and function as a baseline for future incidents to differentiate between acute and unresolved old emboli. This resolution is dependent on the age of the patient, with complete resolution in young age-groups and less complete and less significant resolution in older age-groups [14, 15]. Other factors include age of the thromboembolus or length of time between formation of the embolus and the institution of proper anticoagulation. This is the basis behind the relatively recent trend of starting anticoagulant therapy in most patients with pulmonary emboli who have no contraindication for anticoagulation immediately when a pulmonary thromboembolus is suspected before finishing the workup for the condition. Anticoagulant therapy may then be stopped if the condition is excluded.
13.2.3.3 Chronic Pulmonary Thromboembolism
Incomplete resolution of acute pulmonary embolism is frequently observed and may rarely result in chronic thromboembolic pulmonary hypertension [16]. Chronic thromboembolic disease is characterized by intraluminal thrombus organization and fibrous stenosis or complete obliteration of pulmonary arteries. The consequence is an increased pulmonary vascular resistance resulting in pulmonary hypertension and progressive right heart failure. Pulmonary endarterectomy is the preferred treatment [17].
13.2.3.4 Recurrence
Pulmonary thromboemboli recur in up to 50 % of patients [15], although the incidence in treated PIOPED patients was only 8.3 % [18]. The vast majority of deaths among pulmonary embolism patients are due to recurrent emboli. In the PIOPED study population, it was found that nine of ten people who died had a recurrent pulmonary embolus [19]. Recurrence has been reported to occur at the same site as the original thromboembolus [20].
13.2.3.5 Diagnosis
The clinical diagnosis of pulmonary thromboembolism is difficult and unreliable, due to the nonspecificity of its symptoms and signs as well as the laboratory and chest X-ray findings [21, 22]. Chest X-ray however must be obtained since it may show many parenchymal diseases and must be available for lung scan interpretation (Fig. 13.7). Pulmonary embolism may also be asymptomatic. In the literature, only 24 % of fatal emboli were diagnosed antemortem (Table 13.2) [23–31]. Furthermore the presentation is commonly more difficult and atypical in older age group compared to younger patients [32, 33]. Accordingly only 24 % of fatal emboli were diagnosed antemortem (Table 13.2) [23]. Data indicate that the mortality of pulmonary embolism is more than 30 % if untreated. Promptly diagnosed and treated, emboli have a mortality of 2.5–8 % [13, 14, 19]. The mortality of PE was found to vary among patients with or without cardiac disease. Paraskos et al. [34] reported survival rates at a mean follow-up period of 29 months of 19 % among patients with prior congestive heart failure and 86 % for those with no prior congestive heart failure. Pulmonary angiography is the most accurate modality for the diagnosis of pulmonary emboli with an accuracy of 96 % [35]. However, angiography is invasive and is not suitable as a screening imaging modality for the disease.
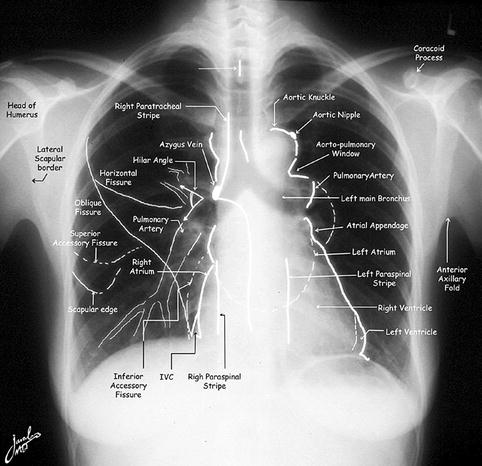

Fig. 13.7
Normal chest X-ray illustrating the important structures that may show variants on perfusion scans
Table 13.2
Antemortem Diagnosis of PE
Author | Year | No. (%) of cases with antemortem PE diagnosis |
|---|---|---|
Stein and Henry [24] | 1995 | 6/20 (30) |
Morgenthaler and Ryu [25] | 1995 | 29/92 (32) |
Morpurgo and Schmid [26] | 1991 | 26/92 (28) |
Sperry et al. [27] | 1990 | 275/812 (34) |
Karwinski and Svendsen [28] | 1989 | 267/1,450 (18.4) |
Gross et al. [29] | 1988 | 7/18 (39) |
Dismuke and Wagner [30] | 1986 | 41/203 (20) |
Goldhaber et al. [31] | 1982 | 16/54 (30) |
Total | 667/2,741(24) |
D-dimer is a fibrin degradation product present in the blood after a thrombus is degraded through fibrinolysis. The blood test to determine D-dimer concentration helps diagnose thrombosis. Although a negative result practically rules out thrombosis, a positive result can indicate thrombosis but does not rule out other potential causes. Its main use, therefore, is to exclude thromboembolic disease where the clinical probability is low.
Scintigraphy
Scintigraphy remains the most cost-effective noninvasive screening modality. The major advantages include its ability to provide regional and quantitative information useful for the diagnosis, as well as for mapping to guide selective angiography if needed for the diagnosis. Spiral CT is useful in detecting central emboli which has become the most commonly used modality in many centers at the expense of scintigraphy although data are still controversial for peripheral emboli [36–39]. Multislice CT was found to have no added value in patients with high-probability V/Q scans and has a comparable diagnostic value with SPECT V/Q scans [40]. CT also as a single study is not cost effective [41]. It also requires the use of iodinated contrast media with its risk of renal failure and ionizing radiation with its risk of cancer induction [42, 43]. Recently it was also found to result in overdiagnosis of pulmonary emboli [38]. MRI pulmonary angiography will play a greater role [44]; however, the use of contrast media is still a shortcoming. In an experimental study, reversible PE was induced by inflating a nondetachable silicon balloon in the left pulmonary artery of five New Zealand white rabbits. MR V/Q scans were obtained prior to, during, and after balloon deflation. High-resolution contrast-enhanced MR pulmonary angiography was also used to confirm the occlusion of the pulmonary artery. Similar to radionuclide ventilation/perfusion technique, acute PE produced a mismatched defect in the MR V/Q scan. MRA verified the occlusive filling defect in the left pulmonary artery. The study suggests that high-resolution MRA and MR V/Q imaging of the lung is feasible and allows comprehensive assessment of pulmonary embolism in one imaging session [44]. Recently, non-contrast MRI has been studied in the diagnosis of PE [45].
Scintigraphy is also valuable in pregnancy. When indicated low activity of 1 mCi (37 MBq) is used for perfusion, if the perfusion study is abnormal, then ventilation and chest X-ray (if not obtained earlier) are obtained as needed. Based upon the available data, there are no apparent short- or long-term consequences to the fetus from the radiation received as a result of diagnostic ventilation/perfusion scintigraphy. For a V/Q scan, fetal dose would mostly come from tracer accumulating in the bladder, with some internal scatter from the lungs. Either Xenon133 or 99mTc agents can be used safely for the ventilation portion of the exam. Xenon103 has the advantage of not being excreted via the urine.
Scintigraphic Agents
Several agents have been used for ventilation (Table 13.3). Every agent has certain advantages and limitations. Xenon 133 (Fig. 13.8) is useful in evaluating obstructive airway disease. Krypton-81 (Fig. 13.9), 99mTc-DTPA (Fig. 13.10), and Technegas (Fig. 13.11) provide the ability to perform ventilation studies after the perfusion, particularly krypton-81. 99mTc-macroaggregated albumin is used for perfusion. For proper interpretation of lung perfusion/ventilation study, chest X-ray must be available and should be obtained within 12 h of the time of the scans.
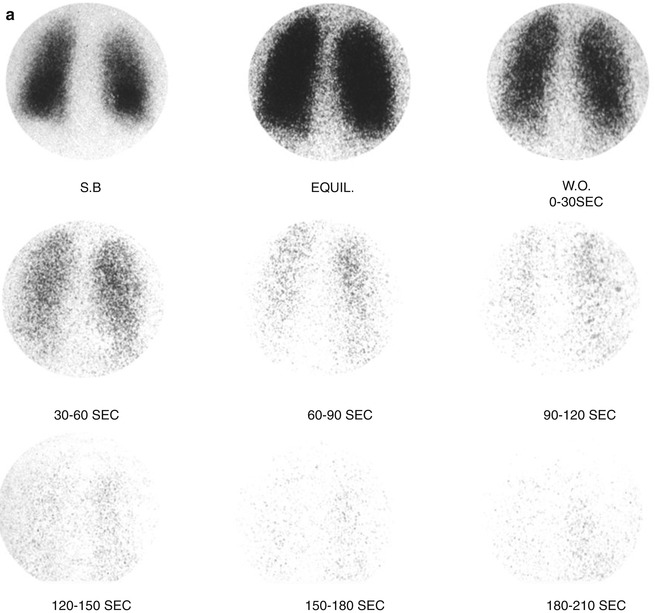
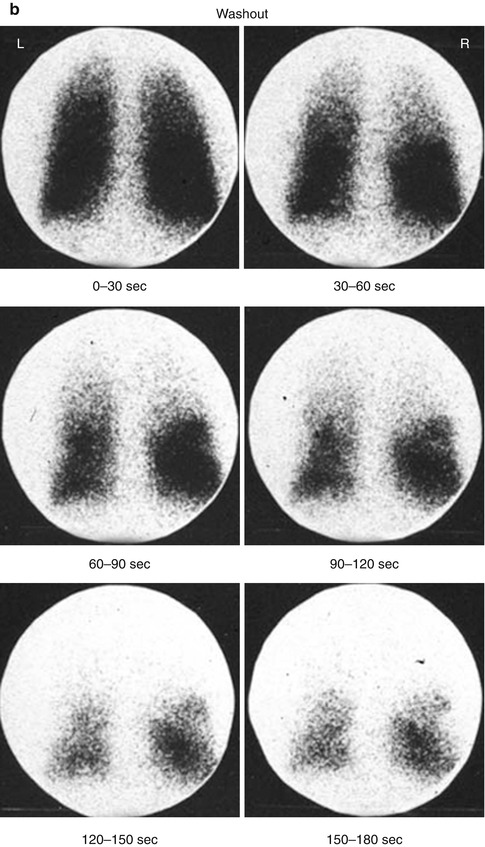
Table 13.3
Ventilation agents
Agent | Advantages and limitations |
|---|---|
Aerosols | |
99mTc-DTPA aerosol | Lung half-clearance time = 58 min |
Pre- or post perfusion | |
Multiple projections | |
99mTc-pyrophosphate aerosol | Post perfusion |
Suitable for SPECT | |
Technegas | Multiple projections |
Good peripheral deposition | |
Gases | |
Xenon-133 | Ability to obtain single breath, equilibrium, and washout images |
Very sensitive for obstructive airway disease | |
Only posterior view is possible in most patients | |
Low energy of 81 keV | |
Pre-perfusion acquisition | |
Krypton-81 m | Expensive – available only in some areas |
Energy: 190 keV | |
Half-life: 13 s | |
Multiple views | |
Pre- or post perfusion | |


Fig. 13.8




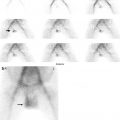
(a, b) Xenon-133 ventilation studies. (a) Normal study with uniform distribution of the radiotracer in both lungs on single breath and equilibrium images. The washout images reveal prompt clearance with no significant retained activity. (b) Washout images of a patient with obstructive airway disease showing retained activity in lower zones of both lungs by the end of the study
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree