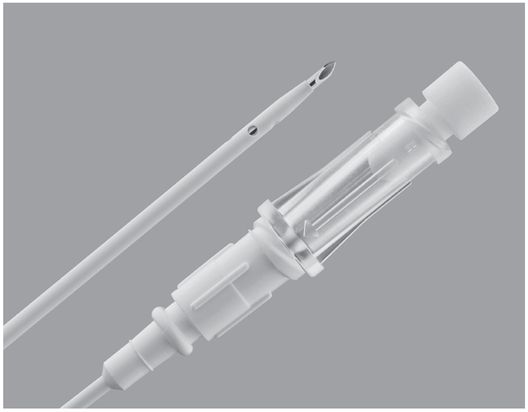
Chiba echo-tip needle (Cook Medical).
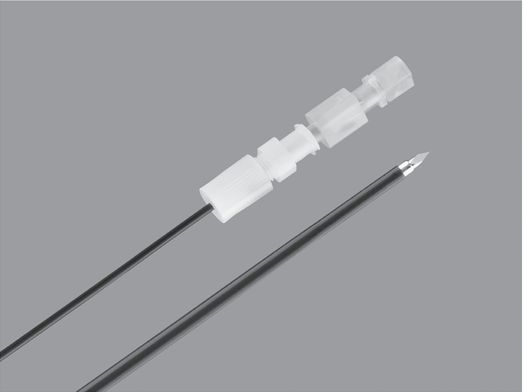
Yueh centesis sheathed needle (Cook Medical).
TFE sheathed needle with trocar stylet (Cook Medical).

“Pigtail” catheters.

Dawson–Mueller Mac-Loc™ 8.5 Fr multipurpose drainage catheter (Cook Medical).

5 French pigtail.
5 and 6 French drainage catheters (Cook Medical).
Patient preparation
Preprocedure imaging
Suspected pleural fluid collections are often suggested on plain film, but a significantly increased yield of important information regarding etiology and complicating factors will be added by CT imaging. The volume and nature of the fluid collection (i.e., free, loculated, or multiseptate; thin or viscous) is often best evaluated using US. It is helpful to identify pleural fibrosis or infiltration to avoid futile attempts at drainage. Dynamic characteristics identified during US imaging can often help distinguish fluid collections from pleural thickening, including flow of particulate debris and flapping septations and atelectatic lung.
Simple transudates often resolve spontaneously and need only be evacuated if they become symptomatic through displacement of lung or mediastinum, or if drainage will prevent repeated thoracentesis. In our experience, stage 2 collections with a complex appearance by US have been easily drained. The re-intervention rate is significantly higher in children with parapneumonic effusions initially treated with aspiration alone when compared to those who receive primary drainage. Unless the collection is known to be a simple transudate, we prefer to place a thoracostomy drain at the initial procedure.
While frontal and lateral chest radiographs are usually the initial diagnostic images obtained and are satisfactory for a diagnosis of pleural and parenchymal abnormalities in most cases, plain film radiography is generally suboptimal for evaluation of mediastinal processes, route planning, and catheter placement. The availability of high-resolution CT and US has led to the widespread use of the percutaneous approach for the management of thoracic fluid collections in children. Computed tomography guidance is preferred for selecting the safest and most direct route and guidance for percutaneous drainage of parenchymal and mediastinal fluid collections. Real-time US guidance is preferred for drainage of pleural fluid collections.
No single imaging modality is used exclusively to guide percutaneous drainage. Whenever possible, real-time US guidance of needle insertion is utilized to gain entry into the fluid collection. Real-time US guidance is also helpful when performing a thoracentesis and for entry into parenchymal abnormalities with pleurodesis since no aerated lung is present to reflect the US beam. Although CT guidance alone is often utilized in the adult population to guide catheter insertion, we prefer to plan the procedure with CT but to guide needle insertion with US and monitor the placement of the guide wire and drains with fluoroscopy.
When CT is required to guide needle insertion, a guide wire is positioned within the abscess cavity and the catheter is inserted blindly over the guide wire (Figure 3.3). In rare instances, the child is transported to the interventional suite where the procedure is completed.
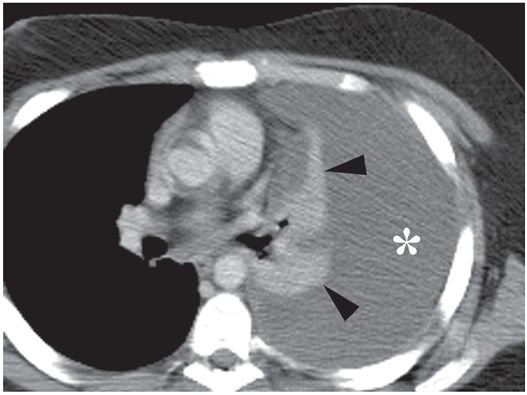
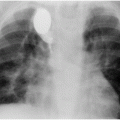
The collapsed lingula and lower lobe (arrowheads) float freely in the large left pleural fluid collection (asterisk) demonstrated on a contrast-enhanced axial CT image through the mid-thorax.
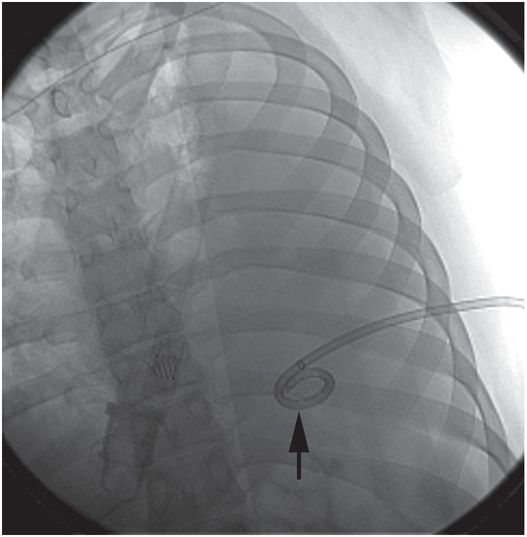
A 14-French multipurpose drainage catheter has been inserted percutaneously into the collection under combined US and fluoroscopic guidance. At the time of insertion, the locked pigtail (arrow) is satisfactorily located in the mid-scapular line in a posterior position; that is, in the most dependent component of the free fluid collection.
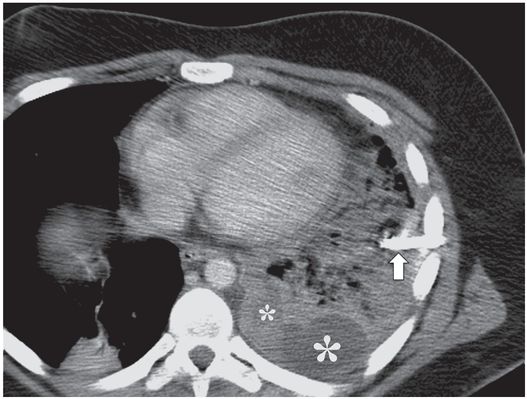
A contrast-enhanced axial CT image shows that the catheter has been partially dislodged and the loop (arrow) is now neither in a gravity-dependent position nor is it within the remaining loculated collections (asterisks). Repositioning alone is unlikely to restore function.
Preprocedural lab studies
Prior to any percutaneous procedure in the thorax, a coagulation profile (PT or INR, PTT, platelet count), and hemoglobin level are obtained. The child is kept NPO for at least four to six hours according to the anesthesia or sedation policy of the hospital. Antibiotics are given if the child is not already being treated.
Standard technique
Management of parapneumonic effusion
When aspiration without drainage of a pleural effusion or empyema is performed to obtain a diagnostic sample or as a means of therapy (usually for collections smaller than 3 cm), the modality selected for guidance depends on operator preference and ease of performance. In most situations a real-time US-guided approach is utilized. Fluoroscopic monitoring is not necessary in most cases, since guide wires and catheters are not being inserted.
If aspiration alone is indicated, for small fluid collections a 20 to 60 ml syringe is attached to the connecting tubing, and the fluid is removed manually. Larger (>500 ml) collections may be evacuated by attaching the connecting tubing via a Christmas tree adapter to either a wall or portable suction device. The suction device is usually set to –10 to –20 cm H2O pressure.
When inserting a drain in a pleural fluid collection, a combination of US and fluoroscopic guidance is used in most instances. The child is usually positioned with the side to be drained elevated 30–45 degrees. Real-time US is used to guide needle access into the fluid collection. After preparing and draping the patient in sterile fashion, an entry site is chosen, usually from the third to tenth intercostal space in the posterior axillary line, with the patient in a supine position. A cooperative older child may be seated at the edge of the procedure table, leaning forward (over a pillow, for example), supported by a member of the interventional team. In this position, the pleural fluid tends to locate in the posterior costodiaphragmatic recess in the region of the eighth to the tenth intercostal space in the mid-scapular line.
The skin is anesthetized with buffered 1% lidocaine, keeping the thin (27- or 30-gauge) needle just superior to the rib to avoid the neurovascular bundle. The course of the access needle may then be observed under US to confirm a safe access route. A small skin incision is made and the subcutaneous tissue is bluntly dissected (Table 3.3). Depending on the size, depth, and location of the fluid collection, an 18- to 23-gauge Chiba needle (for central or deep collections) or a 19-gauge sheathed needle (for superficial or large collections) is inserted into the fluid collection.
1. Route planning.
2. Coagulation profile prn.
3. Sedation or anesthesia for procedure.
4. Prepare, drape, locally anesthetize.
5. Insert needle into abscess using image guidance.
6. Insert guide wire.
7. Dilate tract to size of the drainage catheter.
8. Insert drain.
9. Secure drain with suture and retention device.
10. Suction collection device prn.
11. Postprocedure chest radiograph.
12. Follow-up contrast injection of cavity prn.
The sheathed access needle is guided into the collection under real-time visualization and, once in the fluid collection, the sheath is advanced while pinning the stylet. Pleural tenting may give the false impression that the parietal pleura has been traversed. A short thrust with the needle will usually assure safe and successful passage. During expiration (or the inspiratory phase of positive pressure ventilation), the stylet is withdrawn and the sheath attached to the Luer lock T-connector. It is our practice to always attach connecting tubing, together with a flow switch (Meditech®), to the sheath or needle hub to avoid inadvertent dislodgment or retraction due to patient movement.
At this point, samples of the pleural fluid are obtained to avoid accidental contamination or forgetting to submit a specimen. As small a sample as necessary for planned laboratory analysis should be obtained so that enough fluid is left to form the loop of a locking drainage catheter. If the aspirate appears non-purulent, the fluid is sent for an immediate Gram stain. If results are significantly delayed, or there is a question concerning the nature of the fluid, or the collection is large, a drainage catheter is left in place until the results of the culture are known or the child is asymptomatic.
If the material obtained is purulent, malodorous, or cloudy, a drain is placed. Intrapleural location of the sheathed needle is confirmed by aspiration of fluid or injection of a small amount of contrast. The distribution of contrast should be evaluated if a loculated or septated collection is suspected. Next, a guide wire is advanced until a substantial amount of the stiff portion of the wire is in the pleural space. If a sheathed needle (e.g., Yueh) is selected for initial puncture of the collection, a 0.035-inch or 0.038-inch guide wire can be placed into the collection directly after removal of the stylet. This approach is taken whenever possible, since it reduces the complexity of the procedure and the time required for completion. The wire may be swept through the collection for mechanical dehiscence of septations to encourage more complete drainage. The sheath may then be exchanged over the wire first for the appropriately sized fascial dilator, then for the drainage catheter. Overdilatation of the tract, useful during drain insertion into abdominal fluid collections, is not recommended for pleural drainage because of the increased risk of pneumothorax, leakage of fluid, and contamination of the pleural space.
The diameter of the internal locking pigtail drainage catheter should be selected based upon the viscosity, or fibrous nature, of the fluid obtained. We have found that it is generally best to use larger drains for treatment of pleural effusions than would be used for drainage of abdominal fluid collections. Simple transudates and uncomplicated exudates are drained through a 10 or 14 French catheter, while very thick collections may most effectively be drained through a large bore catheter such as the Thal-Quick. When intracavitary fibrinolysis is employed, the viscosity of the fluid can be expected to decrease substantially in most cases. Once in place, the catheter should be advanced and then fed off the stiffener until the last side-hole is within the pleural space. After the stiffener and guide wire have been removed a small amount of contrast may be injected to confirm intrapleural placement. Proper location in two imaging planes should be confirmed if fluid is not freely obtained or a complication is suspected. Careful attention should be directed to the possible demonstration of a sinogram during contrast instillation, indicating a bronchopleural fistula. The catheter can be secured using a variety of methods. Currently, we use a SorbaView® dressing and may also suture the drain in place. Alternatively, we have found the StatLock® covered with an occlusive dressing (such as Tegaderm® or Opsite®) is also satisfactory. In small infants, we may use a tape bridge (Figure 3.4) covered with a transparent dressing to secure a catheter since it can be easily tailored to size and is more comfortable for the patient. The Thal-Quick catheter may be secured with a subcutaneous purse-string suture, wrapping the free ends of the suture around the catheter at the skin exit site before tying. This suture may be used to close the exit wound when the catheter is removed.
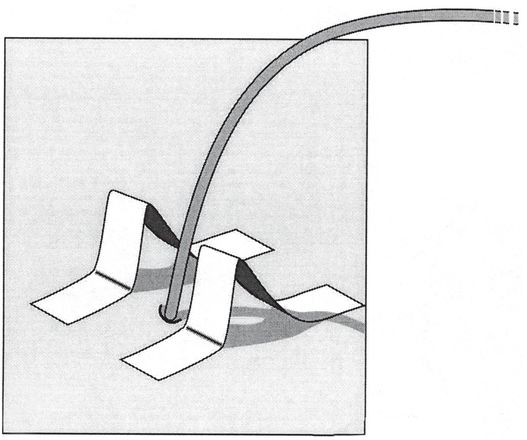
The skin is prepared with mastisol to enhance tape adhesion. Then two pieces of tape of the same length are folded at the halfway point. The tape is stuck together for half of its length. The legs are created by folding the final quarter in opposite directions. The legs are then adhered to the skin so the pieces form “goal posts” on either side of the catheter exit site.
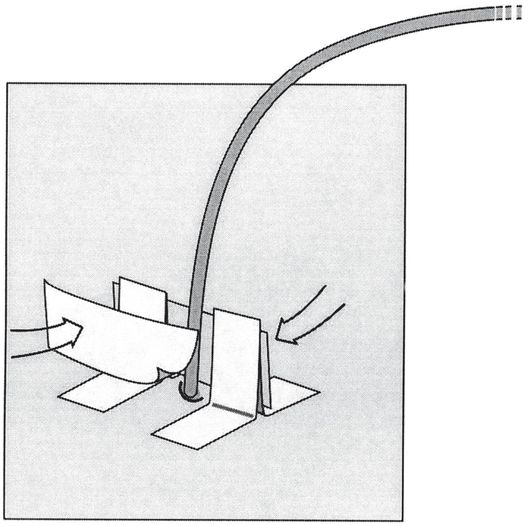
The uprights and drainage catheter are then joined by a bridging piece of tape on both sides (arrows). Any tug on the catheter is now transferred through the tape bridge to the skin, helping to prevent accidental dislodgment.
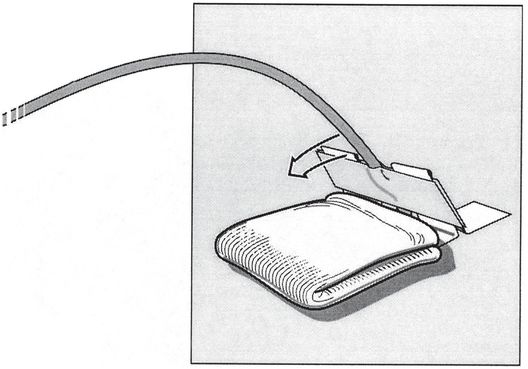
The adjacent skin is padded with gauze and the tape bridge is folded down (arrow). The direction must be consistent with the original angle of entry of the access needle, or the catheter will tend to kink.
The tape bridge is secured to the skin with adhesive tape (arrow).
After securing the drainage catheter, it should be immediately connected to an underwater sealed vacuum drainage system, such as the Pleur-evac®, at –20 cm H2O pressure. In the absence of respiratory distress or concern for electrolyte or fluid shifts, large collections should be slowly drained (in aliquots not exceeding 60 ml/kg) to avoid electrolyte imbalances or re-expansion pulmonary edema. If re-expansion pulmonary edema occurs, it should be treated with supplemental oxygen and diuretics.
Postprocedure and follow-up care
Catheter care
When the pleural space is drained, the catheter is attached to a closed drainage system and placed to continuous suction or gravity drainage, depending upon the type of fluid encountered. When the fluid is thick or viscous, the Pleur-evac® is set at approximately –20 cm H20, and continuous suction is applied. Pertinent details of the procedure are recorded in the patient’s chart, and orders entered (e.g., regarding management of the catheter and any suction devices). It may be helpful to both patient care and ongoing quality assurance to maintain pertinent demographic and procedure-related data in an electronic database. Suggested items for data management are included in Table 3.4. It is our practice to observe a child for at least two hours after a chest procedure on an outpatient basis to make sure that no complication has occurred. In addition, anteroposterior and lateral chest radiographs are obtained to confirm the absence of complications before discharge. The same routine is performed for asymptomatic inpatients.
| To improve outcome and quality assurance analysis, it may be helpful to record the following information for each thoracic interventional procedure performed: – Demographic information (patient name, unique identification number, date and time of procedure, age, sex, weight, date of birth, etc.) – Primary diagnosis – Reason for procedure; referring service and provider – Anticipated endpoint – Primary provider responsible for procedure (interventionalist, pulmonologist, surgeon, etc.) – Collaborating provider(s) – Procedure location (interventional suite, operating room, ICU, bedside, etc.) – Provider(s) responsible for anesthesia/sedation (interventionalist, anesthesiologist, nurse, etc.) – Preprocedural evaluation (e.g., coagulation profile, pulmonary function studies) – Preprocedural interventions (e.g., antibiotics, blood products, imaging) – Initial access access needle guidance (anatomic landmarks, US, fluoroscopy, CT, MRI, etc.) operative site device (e.g., APD, Dawson-Mueller, Thal-Quick, Palmaz® stent, etc.) number of attempts reason for deferral, discontinuation or failure, if procedure not completed – Access drainage device and position manufacturer, type, diameter, length tip position – Equipment and supplies used – Procedure time – Type and adequacy of anesthesia/sedation/analgesia – Procedural complications (e.g., pneumothorax, bleeding) and management – Adjunctive procedures (e.g., intracavitary tPA, dose, dwell time) – Complications (major or minor), including 1. Procedure-related discomfort (include dates) a. type b. management (e.g., NSAIDs, narcotics) c. outcome 2. Pulmonary complications (include dates) a. type (e.g., air leak, fistula, dissemination of pathology) b. method of diagnosis c. management (e.g., observation, intervention, surgery) d. outcome 3. Bleeding and vascular injury (include dates) a. type (e.g., hemorrhage, hemothorax, arteriovenous (AV) fistula, or pseudoaneurysm) b. method of diagnosis (e.g., hemoglobin, CT scan, arteriogram) c. management (e.g., observation, transfusion, embolization) d. outcome 4. Dislodgment or malposition (include dates) a. method of diagnosis or documentation b. management c. outcome – Other procedure-related complications or interventions – Outcome at follow-up (method and date; endpoint achieved?) postprocedural lab studies (e.g., fluid pH, culture, or biopsy findings) response (e.g., reduction in fever, dyspnea, debilitation, discomfort) results (e.g., daily volume of fluid drained, radiographic evidence of resolution, hospital days) treatment failure (e.g., residual pleural “peel”, recurrent pneumothorax) change in pulmonary function |
If an associated bronchopleural fistula or pneumothorax is present, a Pleur-evac® is used with suction set to –20 to –30 cm H2O to aid tract closure. Larger air leaks may require a larger chest tube (such as an 18 to 22 French Thal-Quick) or multiple tubes to successfully evacuate the air leak. If closure cannot be achieved after several weeks of drainage, the surgical service is consulted. Irrigation of the catheter is performed at least twice per day when thick or viscous fluid is noted but not routinely for thin fluid. In addition, intracavitary thrombolytic agents are considered as an adjunctive therapy when drainage is incomplete, or the fluid is thick, viscid, loculated, or fibrinous (see “Adjunctive transcatheter therapies in the thorax,” below). The catheter is left in place until all significant drainage has stopped (less than 10 ml/day), and resolution or significant improvement of signs and symptoms has been achieved.
An important and desirable endpoint of therapy is re-expansion of the associated lung and re-establishment of normal pulmonary function. Failure to observe the lung re-expand may indicate restriction by an organized pleural peel, intrinsic lung disease, presence of an endobronchial lesion, ineffective drainage (Figure 3.3d), or airway compression by an extrinsic lesion.
Postprocedural lab studies
Because the fluid obtained at thoracentesis or thoracostomy tube placement is sampled from an otherwise normally sterile space, examination of the fluid may provide information necessary to make a diagnosis of the underlying abnormality. This sample should be handled with care, without inadvertent contamination or introduction of bacteriostatic agents (i.e., through mixture with bacteriostatic saline or contrast). A Gram stain of the sample should be performed and aliquots distributed to the microbiology laboratory for culture (e.g., aerobic and anaerobic bacterial, tuberculosis and fungal cultures), cell count, and differential analysis. Biochemical analysis should include pH, glucose, lactic dehydrogenase (LDH), protein, and amylase. Simultaneous serum pH, glucose, LDH, and protein should be determined. In the proper clinical setting, rheumatoid factor and antinuclear antibody titers may be helpful. Additional special lab studies may be indicated in specific clinical situations (e.g., adenosine deaminase and gamma-interferon in suspected TB; pleural fluid cytology in suspected pleural malignancy).
Complications
As with any drainage procedure, mechanical dysfunction is not uncommon, including catheter kinking, accidental dislodgment (Figure 3.3), iatrogenic suture ligation of the catheter, and inadvertent closure of valves or stopcocks. In our experience, serious complications occur in less than 2% of cases. The main risks of aspiration and drainage of the thorax include symptomatic pneumothorax, hemothorax, pyothorax, pyopneumothorax, pulmonary hemorrhage, injury to intercostal and other vessels, and contamination of the pleural space. Pleural contamination may occur when draining a lung or mediastinal abscess in the absence of pleurodesis. In spite of these risks, serious untoward effects infrequently occur.
Pneumothorax
Free communication between the pleural space or pulmonary perivascular space and the atmosphere, either from alveolar or chest wall trauma, allows for the development of air collections in the pulmonary interstitium (pulmonary interstitial emphysema), the hilum and mediastinal space (pneumomediastinum), or the intrapleural space (pneumothorax). From these spaces, air may dissect into the peritoneal cavity (pneumoperitoneum), the soft tissues (subcutaneous emphysema), or the pericardium (pneumopericardium). Massive continued air leak may lead to elevation of the pressure (tension) in the air-containing space. This may produce compression of vital structures, including the lungs, heart (tamponade), and major vessels, with concomitant cardiorespiratory compromise. Measures taken to improve ventilation in these patients often exacerbate the tension. Children with a small or asymptomatic air collection may be treated with 100% O2 (“nitrogen washout”). This will produce a differential partial pressure in the air-containing space that should hasten its reabsorption, in the absence of continuing air leak.
In the face of a clinical situation consistent with tension pneumothorax with cardiorespiratory compromise, immediate decompression is essential. This is most easily accomplished empirically with a 16- to 20-gauge angiocatheter or needle advanced perpendicularly through the skin just superior to the second rib in the midclavicular line, without prior imaging or image guidance. Prompt radiographic confirmation should be sought after this procedure. If the patient is stable enough for preprocedure imaging, or if emergent needle aspiration is positive for air or fluid, a chest tube should be placed in the interventional suite under fluoroscopic guidance, using the same technique outlined above for drainage of pleural fluid. Percutaneous catheter placement enables safe and effective drainage of pneumothoraces with rapid restoration of vital capacity, oxygenation, and lung re-expansion. The catheter should be connected to an underwater seal and suction applied at –5 to –15 cm H2O. Where loculated or multiloculated pneumothoraces or pneumatoceles are unstable or cause cardiopulmonary compromise, especially those located in regions technically difficult to reach by conventional approaches, CT guidance may offer a safe and accurate alternative for percutaneous placement of small-bore thoracostomy catheters.
Hemothorax
In the face of frank bleeding at the time of thoracostomy tube placement, transcatheter embolization or direct surgical repair of bleeding vessels should follow immediate vascular volume expansion. Most often, children developing a hemothorax require chest tube drainage to decompress the pressure effects and reduce the chance of future obliteration of the pleural space.
Chylothorax
Accumulation of chyle in the pleural space may cause life-threatening cardiopulmonary complications. This should be treated with immediate thoracentesis, repeated as necessary. Often, chylothorax will respond to a single thoracentesis with complete evacuation, followed by medical management directed at reducing chyle production and supporting nutritional losses. Direct surgical intervention may be avoided by embolization of the thoracic duct and any major collaterals guided by fluoroscopic pedal, or direct nodal, lymphangiography and cannulation of the cisterna chyli. Alternative methods of draining the thoracic duct to a neck port or to the esophagus are being explored in animal models. Surgical therapy may also be considered.
Conclusions
Image-guided aspiration and drainage is the treatment of choice for most abnormal fluid collections involving the pediatric thorax, eliminating or markedly reducing the need for open surgical or thoracoscopic intervention. With the use of image guidance, needles and catheters can be accurately and safely inserted into fluid collections in all compartments of the thorax. Adjunctive therapies, such as intracavitary fibrinolysis or pleurodesis (see below), further expand the scope of problems amenable to image-guided therapies. As is the case for other anatomic sites, percutaneous drainage may be used in lieu of or as an adjunct to surgical drainage. The use of these interventional techniques will likely reduce postprocedural morbidity, shorten hospital stays, and decrease costs in the pediatric population.
Adjunctive transcatheter therapies in the thorax
Introduction
The insertion of a drainage catheter in the pleural space or within a pathologic intrathoracic cavity (e.g., pulmonary abscess or necrotic tumor) provides the means for introduction of various adjunctive therapies. While simple pleural effusions can easily be treated with catheter drainage, complex pleural collections may require more intensive therapy, including instillation of fibrinolytic agents, in order to break up fibrinopurulent barriers and to decrease the viscosity of the fluid component. Similarly, a simple pneumothorax may be satisfactorily treated by suction applied through a catheter. However, patients with recurrent pneumothoraces may require more definitive therapy, such as chemical pleurodesis. Intracavitary chemotherapeutic treatments, such as delivery of antifungal and antineoplastic agents, are also possible using a preexisting catheter. In each case, successful adjunctive transcatheter therapies may avoid more invasive and costly alternatives. Intracavitary fibrinolysis serves as a prototypical transcatheter therapy and will be discussed in detail. Other transcatheter therapies will be briefly introduced.
Image-guided therapy, using US, fluoroscopy, or CT, alone or in combination, permits definition of, access to, and drainage of free or loculated empyemas with a high rate of success and substantially reduced risk. Preprocedure imaging, whether plain radiograph, CT, or US is poorly predictive of the likelihood of success of catheter drainage alone, although US provides the most accurate evaluation of the nature of the parapneumonic collection (anechoic, complex non-septated, or complex septated). Selecting the best therapeutic strategy for children with empyemas is a controversial decision and is often dependent on individual experience and preference. Although antibiotic therapy has significantly reduced the incidence of empyema associated with pneumonia, 50% of all empyemas are still a complication of bacterial pneumonia.
Adjunctive therapy with fibrinolytic enzymes may be used to decrease the viscosity of almost any type of proteinaceous collection and to improve drainage of loculated collections, thick pus, or blood. In 1949, Tillet and Sherry used streptokinase to treat fibrinous pleurisy, bacterial empyema, and hemothorax. One year later, Sherry and associates described the use of streptokinase and streptodornase for treatment of loculated hemothorax and empyema. Since then, many others have reported the use of streptokinase for the treatment of empyema, thereby avoiding a thoracotomy. However, the use of intracavitary thrombolytic agents did not gain favor until Vogelzang and colleagues reported the first use of urokinase (UK) for the treatment of infected extravascular hematomas. Hemorrhagic complications with intracavitary fibrinolysis have been reported, but are rare. In 1989, Moulton and associates reported their experience with intracavitary UK for the treatment of loculated pleural collections. This report and others have led to the use of adjunctive fibrinolysis in the pediatric population. When UK was temporarily removed from the market, other thrombolytic agents, especially tPA, gained widespread use for intracavitary thrombolysis. Our experience and that of others with the intrapleural instillation of fibrinolytic agents has shown that it is a good alternative to surgery during the acute phase, prior to organization of the pleural fluid collection.
Multiloculated fibrinopurulent empyemas and fibrinous effusions are difficult to treat because of the difficulty lysing the internal fibrin septations. In the past, this limited the efficacy of tube drainage, often necessitating operative intervention or the insertion of large-bore chest tubes. When percutaneous drainage with smaller tubes (≤ 8 French) was performed and septations were identified, the interventionalist would try to mechanically lyse the septations by maneuvering a guide wire within the pleural space, flushing with saline to unplug the catheter, or inserting multiple drains into the major loculations. In spite of these approaches, complete and prompt drainage of complex collections was often not accomplished, leading to prolonged hospitalization with or without surgery for decortication. Currently, tPA is the lytic agent of choice and is well tested with good results in children.
Indications
In the pediatric population, the indications and contraindications for the use of intracavitary thrombolytic agents are somewhat arbitrary since limited prospective studies have been performed. In our opinion, the use of fibrinolytic therapy (Figure 3.5) is indicated during stage 1 of a pleural effusion, especially if there is fibrinous pleural fluid, hemothorax, collections draining more slowly than anticipated, thick or viscid fluid collections, or children in whom imaging suggests thin septations within the pleural space (Light stage 2) (Table 3.5). Patients known to have a thickened visceral pleural peel, pleural fluid pH <7.2, LDH >1,000, or glucose <40 mg/dl may be considered for early referral for surgical management although some pediatric interventionalists recommend that all children, regardless of the stage, be treated with percutaneous drainage and those with stage 2 or 3 effusions also receive a trial of intracavitary fibrinolysis. Then, only those children who fail therapy should be referred for VATS. At this time, there are no prospective studies to turn to for guidance.
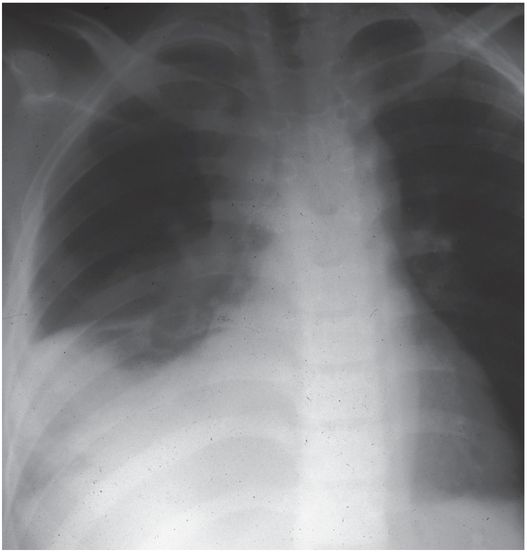
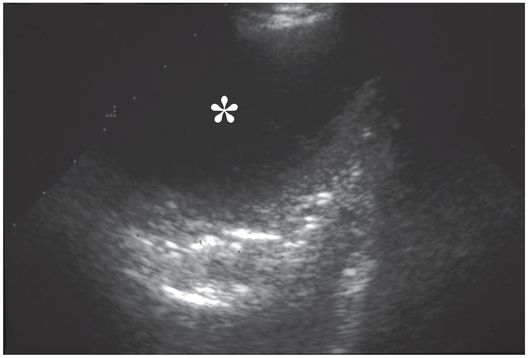
Ultrasound examination prior to thoracostomy tube drainage assisted by fibrinolysis reveals a 10.9 cm loculated fluid collection (asterisk) that is anechoic except for subtle internal septations.
| Indication: 1. For drainage of a pleural (parapneumonic) fluid collection, if: a. acute collection (Light stage 1), or b. fibrinopurulent collection (Light stage 2) c. trial of therapy for organized collection (Light stage 3) 2. To decrease viscosity of thick pus or blood, including infected hematoma 3. To assist drainage of complex, loculated, or septated collections 4. Treatment of infectious or neoplastic disease, e.g.: a. bacterial or fungal infection – antibiotic or antifungal b. malignant effusion – antineoplastic c. echinococcal cyst – antiparasitic and sclerosant d. thoracic lymphocele – sclerosant |
| Contraindications: 1. Uncorrected coagulopathy or known bleeding diathesis 2. Frank blood return from chest tube, or recent intrapleural or intracavitary hemorrhage 3. Major thoracic or abdominal surgery within ten days 4. Hemorrhagic stroke, intracranial neoplasm, cranial surgery or head trauma within 14 days 5. Active or recent bronchopleural fistula 6. Thickened visceral pleural peel (Light stage 3) |
Intracavitary thrombolytic therapy may be contraindicated in children with a known sensitivity to the fibrinolytic agent, recent intrapleural or intracavitary bleeding, children who have had major thoracic or abdominal surgery within ten days, a history of hemorrhagic stroke, intracranial neoplasm, cranial surgery or head trauma within 14 days, and in the presence of a bronchopleural fistula, to avoid recurrent hemorrhage or reopening of the fistulous connection. Moulton and colleagues recommend at least a three-day wait before intracavitary instillation of thrombolytic agents after an intrapleural bleed. Although we have worried about the potential of systemic effects, several studies have suggested that intracavitary instillation of fibrinolytic agents does not result in a systemic fibrinolytic effect. To date, we have not identified adverse effects resulting from intracavitary fibrinolysis of complicated pleural effusions in children. Also, there has been no prospective study to investigate the incidence of uncovering a bronchopleural fistulae with fibrinolytic agents, although we have seen instances of this complication.
Chemical pleurodesis is indicated to treat chronic or recurrent air leaks, such as occur in patients with recurrent spontaneous pneumothorax, cavitary pneumonias, or chronic obstructive pulmonary disease. It may also be useful in patients with symptomatic malignant pleural effusions that have previously responded to thoracentesis. A similar approach has been described for ethanol sclerosis of pericardial cysts. Directly instilling antifungal agents into the pleural space or cavity may be considered when fungal pneumonias are associated with evidence of chest wall, cavitary pulmonary disease, or pleural involvement. For example, amphotericin B has been instilled in the pleural space for treatment of aspergillus and pulmonary mucormycosis, with variable results.
Technique
Equipment
With a preexisting intrapleural catheter in place, no special equipment is required for adjuvant therapies.
Patient preparation
If the use of inflammatory agents is contemplated during intracavitary therapy, pain management specialists may be of assistance during and following the procedure in order to avoid unduly severe or prolonged patient discomfort.
Standard technique
Intracavitary fibrinolysis
Our current approach to intracavitary fibrinolysis in children follows the approach described in the adult literature, as modified for children (Table 3.6). Given the intermittent availability of UK, we now use tPA, 5 mg dissolved in 25 to 150 ml sterile saline, as appropriate to the volume of the pleural space. If UK is used, up to 250,000 U may be dissolved in 250 ml sterile normal saline. The optimal volume of fluid injected appears to relate to distribution of the agent in the pleural space. Therefore the unit dose of fibrinolytic should be delivered in a volume sufficient to achieve adequate distribution without compromising cardiopulmonary function.
1. Chest radiograph or chest CT for diagnosis and planning
2. Sedate or anesthetize child
3. Chest drain insertion
4. Inject tPA 2 to 5 mg in 50 to 150 ml of normal saline depending on patient size
5. Clamp drain for one to three hours
6. Gravity drainage, suction device, or wall suction at –20 cm H2O
7. Follow-up chest radiograph or chest CT
8. Repeat treatment as needed or use twice-daily approach
After instillation of a volume appropriate to the size of the patient, divided among the drainage tubes if multiple loculations are being treated simultaneously, the chest tube is clamped for a minimum of one hour during which time the patient is asked to change position frequently in order to bathe the entire pleural surface in the solution. Fluid is then aspirated, the net output recorded, and the catheter reopened to suction drainage. After one hour of drainage (–20 cm H20) the process may be repeated two to four times daily. Although some grossly bloody output may be observed without clinical significance, active bleeding into the pleural space is considered an absolute contraindication to intracavitary fibrinolysis.
Chemical pleurodesis
Chemical pleurodesis provides a means to treat recurrent pneumothoraces or persistent air leak when conventional therapy has failed. This often includes children with spontaneous pneumothorax as well as pneumothoraces secondary to a variety of conditions such as congenital cystic lung disease, barotrauma, diffuse lung disease, connective tissue disease, and some metastatic neoplasms. Spontaneous pneumothorax has a high rate of recurrence in patients over nine years of age. Because children under nine years of age have a lower rate of recurrence, they may be safely managed conservatively. Tube thoracostomy offers a minimally invasive therapy for simple pneumothorax but is less often effective in preventing recurrence of spontaneous pneumothorax and its related complications (e.g., air leak, contralateral spontaneous pneumothorax, and tension pneumothorax). Definitive therapy involves obliteration of the potential space by obtaining permanent apposition of the two pleural layers (i.e., pleurodesis). The same approach can be applied to recurrent pleural effusions refractory to other forms of treatment. This may be achieved by a variety of methods including open thoracostomy with apical pleurectomy, mechanical pleurodesis (gauze or brush abrasion), pleural irritation with laser or cautery, or chemical pleurodesis via instillation of an inflammatory agent into the pleural space.
Open surgical pleurodesis is associated with significant morbidity, including prolonged hospital stay, bleeding, and chronic postoperative pain. In the past decade, VATS has become an alternative to open thoracotomy for the performance of mechanical pleurodesis. Closed tube drainage with transcatheter instillation of chemical sclerosants offers a minimally invasive alternative for the prevention of recurrent pneumothorax or effusion and has been performed using a variety of inflammatory agents, including talc, doxycycline, bleomycin, acromycin, tetracycline, OK-432, autologous blood patch, silver nitrate, olive oil, fibrin glue, and other agents. Of these, talc has been the most effective agent in our experience. Approximately 2 to 4 g of sterile talc may be suspended in 100 ml normal saline and injected into the pleural space. Alternatively, 500 mg doxycycline may be mixed in 100 ml normal saline, or 60 IU bleomycin may be mixed in 100 ml of 5% dextrose in water (D5W) and the solution instilled into the pleural space. The pleurodesis agent is allowed to remain in the pleural space for one hour, after which the catheter is returned to water suction for at least 24 hours.
Re-administration on the second and third treatment days is frequently required. The pleural space should be prepared by pre-administration of 20 ml of 0.25% bupivacaine into the pleural space, 20 to 30 minutes prior to instillation of the pleurodesis agent. Deep sedation or general anesthesia is required during the period so that the pleurodesis agent remains in the pleural space, as the resulting inflammation can be acute and painful. Once the agent is evacuated, the painful stimulus usually recedes quickly. No single method or agent is perfectly reliable, but the recurrence rate following pleurodesis by whatever method is significantly lower than thoracostomy without pleurodesis. Transcatheter chemical pleurodesis can be as effective, safe, and rapid as surgical alternatives, especially in patients with bullae <2 cm in diameter. Long-term sequelae may include recurrence, pain, pleural thickening, and fibrothorax.
Postprocedure and follow-up care
It may be helpful to both patient care and ongoing quality assurance to maintain pertinent demographic and procedure-related data in an electronic database. Suggested items for data management are included in Table 3.4. The result of treatment is monitored by chest radiographs and intermittent chest CT. A chest CT is useful in gauging the effects of therapy. If loculated fluid remains, the procedure may be repeated, additional drains inserted, or the patient may be referred for surgical debridement. No studies are yet available that outline the optimal approach to therapy. If no significant residual remains, the patient would be maintained on suction until they were asymptomatic with less than 10 ml of drainage over a 24-hour period. At this point the chest tube(s) are removed.
Complications
Potential complications associated with adjunctive therapy usually relate to the primary procedure (i.e., catheter insertion). Bleeding complications are rare, but fibrinolytics should be held in the face of frank bleeding or recent surgery. Occasionally, intracavitary fibrinolysis may uncover a previously occult bronchopleural fistula. Treatment failure may be seen in late stage 2 and stage 3 disease, including the inability to evacuate viscid material and intractable pleural thickening. These children may best be treated by early referral for surgical intervention.
Conclusions
Clearly, a delay in the definitive therapy of childhood empyema carries a significant risk for an unfavorable outcome, including recurrent empyema with lung abscess, scoliosis, restrictive lung disease, bronchopleural fistula, sympathetic pericardial effusion, and septicemia. However, it remains unclear which definitive therapeutic intervention, catheter drainage with early intracavitary fibrinolysis, VATS, or thoracotomy and decortication of both the parietal and visceral pleural peel, will prove to optimize outcome, cost, and resource allocation. In our opinion, aggressive closed chest interventions with early adjunctive intracavitary fibrinolysis should be attempted in parapneumonic effusions up to four to six weeks old. Later than this, it is likely that chronic inflammation and mature fibrous thickening (stage 3) will require VATS or other surgical approaches.
The dosage of fibrinolytic drug is arbitrary, and few prospective trials have been performed in either adults or children. Therefore the adult dose is usually used and either the volume of fluid infused is reduced proportional to patient size, thereby reducing the total drug dose, or the total drug dose is given in a smaller fluid volume. The latter approach is more frequently used in organizing effusions or those resistant to treatment. Catheter drainage with intracavitary fibrinolysis may obviate need for thoracoscopic or surgical pleural drainage techniques.
Intrathoracic abscesses
Introduction
Before the advent of antibiotic therapy, a lung abscess was a common outcome of pneumonia in children. Prior to the availability of percutaneous management, pulmonary abscess in children with pneumonia or other preexisting conditions caused greater than 90% mortality. With advances in antibiotic therapy, it has become a rare complication, most commonly related to Staphylococcus or Haemophilus influenzae. In an otherwise healthy child, pulmonary abscess is usually a solitary lesion, tending to occur early in the course of disease while the patient is clinically ill. Multiple scattered abscesses are usually due to septic emboli from bacterial endocarditis. The high prevalence of immunologic impairment in children presenting with a pulmonary abscess increases the risk of a poor outcome and the urgency for prompt intervention.
Complications of an untreated lung abscess may include compression of vital structures, cardiorespiratory compromise, spontaneous rupture with spread of infection to other parts of the lung, disseminated sepsis, and death. Chest CT is usually helpful in the identification of multiple abscesses, in the distinction of lung abscess from emphysema, in the characterization of the intrinsic nature of the abscess and its proximity to the pleura, and in the evaluation of a safe access window for percutaneous drainage. Good results have also been achieved in the percutaneous treatment of mediastinal, chest wall, and intraparenchymal abscesses. Most loculated abscesses (Figure 3.6), regardless of their location, require some form of drainage. In older children, lung abscesses often respond to chest physiotherapy or to transbronchial aspiration. However, children less than seven years of age are unlikely to drain spontaneously and require more aggressive therapy.
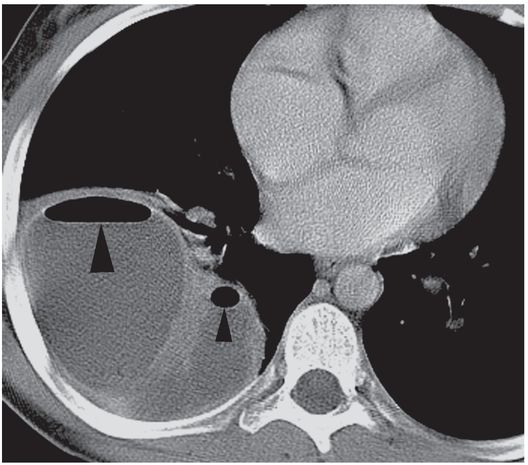
This 12-year-old girl presented with a five-day history of fever and cough. A contrast-enhanced axial CT image of the thorax demonstrates a multiloculated right middle lobe abscess containing non-dependent gas collections (arrowheads).
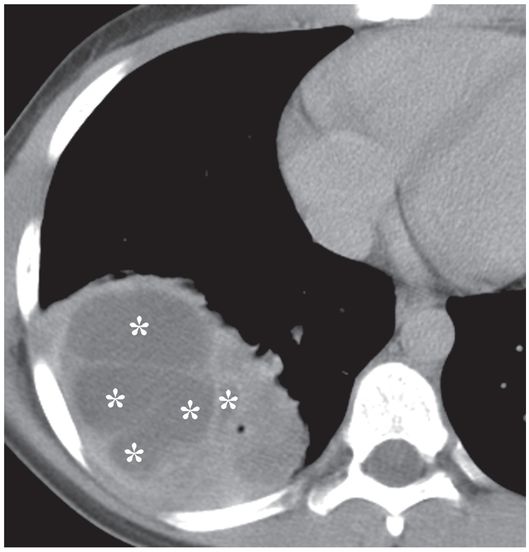
A second axial CT image through the same collection suggests that multiple internal septations divide this collection into several loculated compartments (asterisks).
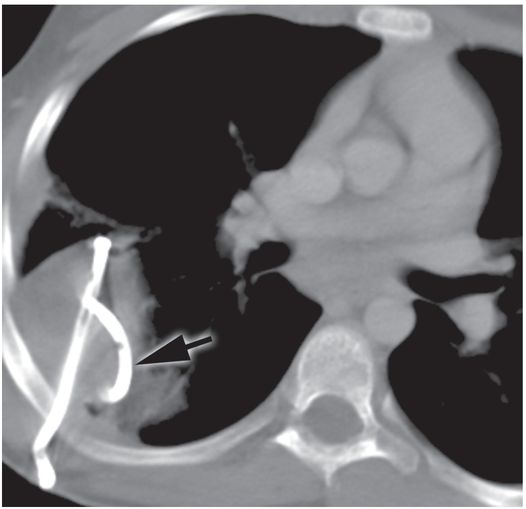
A postprocedure supine axial CT image demonstrates partial evacuation and collapse of the cavity. Despite its loculated appearance, mechanical disruption of the internal septations allowed the entire collection to be successfully drained through a single locking pigtail catheter (arrow). Samples obtained at the time of insertion grew non-typable Haemophilus influenzae. The 10 French drainage catheter was removed on the fifth postprocedure day.
Successful percutaneous diagnosis and treatment of intrapulmonary abscesses can be achieved in children. Lacey and Koslosky have described the use of a local pneumonostomy (mini-thoracostomy) to drain peripheral lung abscesses in children who have failed to respond to medical management. Their success was aided by the presence of pleurodesis (fusion of the visceral and parietal pleural layers) adjacent to the abscess. Since approximately 90% of lesions abut a pleural surface, pleurodesis often facilitates percutaneous drainage of lung abscesses. Creation of the catheter tract through a region of pleurodesis prevents inadvertent contamination of the pleural space and progression to empyema and minimizes the risk of a pneumothorax. Centrally located intraparenchymal abscesses still represent a management problem and may require a thoracotomy and wedge resection for treatment. With the precise anatomic localization available with CT, these collections can now be considered for percutaneous therapy in most instances. Each case should be considered individually and the treatment tailored to each situation.
Indications
As in adults, abscess formation in children most commonly results from aspiration of mouth flora. However, lung abscesses in younger children are less likely to drain spontaneously or to respond to antibiotics. The presence of congenital or acquired immune disorders, impairment of normal protective mechanisms, prematurity, endocarditis, cerebral palsy, poor oral hygiene, or congenital pulmonary abnormalities such as sequestration may result in a predisposition to chronic or recurrent aspiration, or to an extensive or severe bronchopneumonia due to staphylococcal or Gram-negative organisms. Arising from ineffective clearance of infective organisms or incomplete treatment of pneumonia, a phlegmonous consolidation of infected lung parenchyma may progress to necrosis and formation of a circumscribed cavity containing necrotic parenchyma and purulent fluid.
Once it drains through a bronchial communication, a mature lung abscess may have the classic imaging appearance of an enhancing (or echogenic) thick-walled (5–15 mm), irregular cavity (2–20 cm in diameter), filled with variable amounts of fluid and air, forming an acute angle with the pleura (Figure 3.7). This appearance is not specific. The differential diagnosis of pulmonary cavities in children includes pulmonary abscess, loculated empyema, traumatic pseudocyst, pneumatocele, echinococcal cyst, solitary (congenital) cyst, adenomatoid malformation, cavitary tuberculosis, cavitating pneumonia (cavitary necrosis), cavitating hematoma, cavitating infarct, foregut cysts, cystadenomatous malformation and sequestration, and lymphangioma of the lung. Under certain circumstances (such as an inability to mount a normal inflammatory response in the immunocompromised patient), a pulmonary abscess may not be surrounded by a thick wall, may not cavitate, or may become grossly distended with air.
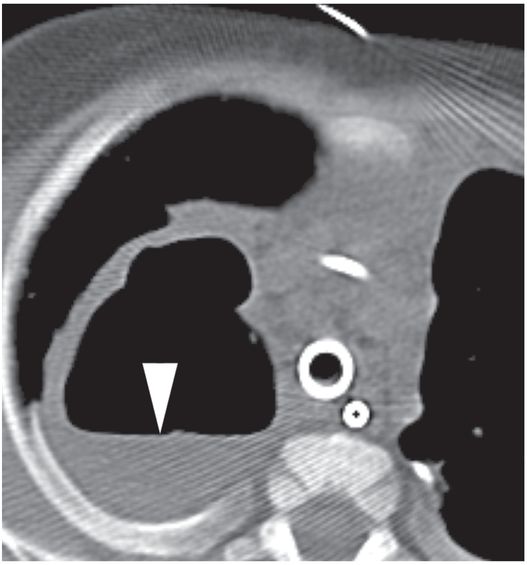
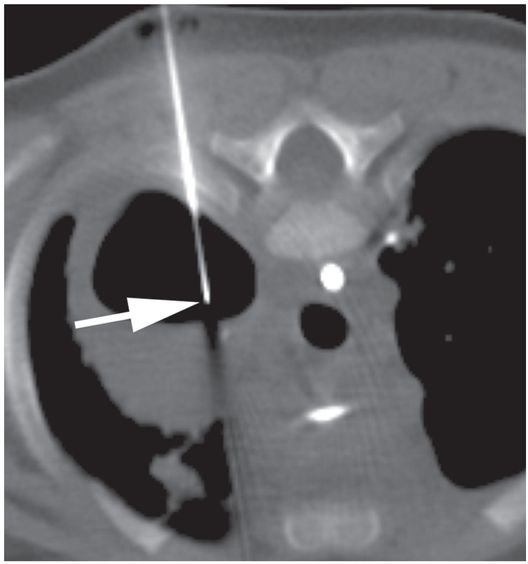
Under CT guidance in a prone position, access to the collection is achieved with a 19-gauge needle (arrow). 2 ml of fluid was aspirated. Microbiologic examination demonstrated Pseudomonas aeruginosa.
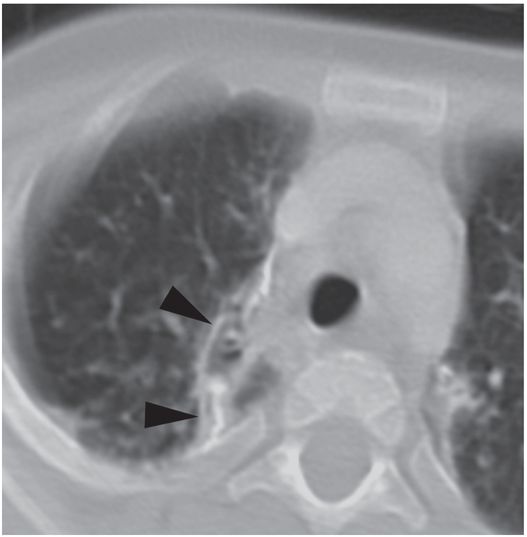
After drainage through an 8.5-French Dawson–Mueller drainage catheter and appropriate antibiotics, a follow-up CT image nine months later shows interval resolution of the bronchopleural fistula and the fluid collection, with residual pleural scarring and calcification (arrowheads).
A parapneumonic pulmonary collection of known bacterial origin and imaging features characteristic of an abscess, as well as a cavity larger than 3 cm in diameter and a location abutting a region of pleurodesis without overlying aerated lung, is an ideal candidate for percutaneous drainage (Table 3.7). Abscesses that are centrally located, multiple, fungal, progress despite catheter drainage, or develop persistent bronchopleural fistula are particularly challenging and may be candidates for a combined interventional and surgical approach, including thoracostomy and surgical resection. Aspiration through a thin (e.g., 18- to 22-gauge Chiba or sheathed) needle (Figure 3.7b) is sometimes considered for diagnosis of a presumed fluid collection of uncertain etiology or for evacuation of a collection too small (i.e., less than 3 cm) to hold a drainage catheter, too deeply located, or with too much overlying aerated lung to be safely drained.
| Indications – Pulmonary abscess (abscesses >3 cm, especially those abutting the pleura or subjacent to pulmonary consolidation) – Chest wall and mediastinal abscesses – Thoracic pancreatic pseudocyst |
| Contraindications – Uncorrectable coagulopathy – Platelet count <50,000 (unable to correct) – Unsafe access route – Fungal disease (controversial) |
As Lee and colleagues demonstrated in a neonatal series, aspiration alone can be curative with minimal risk. However, aspiration alone is substantially less likely to be curative in infected collections large enough to accept the pigtail of a drainage catheter. Unless they can be shown to interconnect, multiple abscesses or multilocular collections may require a separate drain for each locule. All collections should be either drained or aspirated and separate samples sent from each for microbiologic analysis to help select the most appropriate antibiotic coverage. If any discrete collections cannot be safely accessed, surgical incision and drainage may be indicated.
Absolute contraindications to abscess drainage include an uncorrectable bleeding diathesis or unsafe route for drainage. In select cases, fluid collections in these patients may be safely aspirated. A suspected fungal abscess, which can be quite invasive, may be considered a relative contraindication to percutaneous drainage, although some advocate for percutaneous evacuation of intrapulmonary mycetomas. Usually, surgically treated fungal abscesses are preferentially resected rather than drained. Local adjuvant therapy with intracavitary instillation of antifungal agents may provide a viable therapeutic alternative in select cases.
Technique
Equipment
The size, nature, and location of the collection must be identified as well as the safety of the access route guide catheter. Equipment for aspiration and drainage of intrathoracic abscesses is the same as that used in other thoracic collections, as presented in Table 3.2. A Seldinger technique is virtually always preferred over the trochar method.
Patient preparation
All patients have a contrast-enhanced CT scan prior to being referred for the procedure. Coagulation profiles and platelet counts are routinely performed in all patients. A platelet count of at least 50,000 is preferred for the procedure. Any coagulation abnormality is corrected if possible. Admission is planned for those patients with a history or laboratory values suggesting abnormal coagulation or with an ASA classification greater than 2. Procedures are performed under deep sedation with local anesthesia or under general anesthesia. General anesthesia is planned when medically indicated or for patients in whom respiratory control is needed for lesion stability. All patients are kept NPO according to relevant hospital sedation and anesthesia policy prior to the procedure.
Standard technique
A contrast-enhanced CT scan of the chest is most likely to demonstrate the number and character of pulmonary fluid collections, their relationship to vessels and other vital structures, and potential access routes (Table 3.8). If a lesion is in the lung periphery US guidance may be considered. Even if US guidance is contemplated, CT localization can define an optimal approach and suggest a likely sonographic window, and then can be used to confirm satisfactory placement at the end of the procedure. It can therefore be useful to have access to US in the CT suite.
1. Preprocedural work-up (include CT with contrast)
2. Route planning. Mark entry site on the skin surface
3. Sterile preparation and draping
4. Locally anesthetize entry site
5. Small skin incision
6. Image-guided (CT or US) puncture
7. Move to fluoroscopy suite if necessary to monitor remainder of procedure
8. Insert guide wire and coil within cavity
9. Dilate tract
10. Insert locking pigtail catheter
11. Secure catheter to patient
12. Apply a dry, sterile dressing
13. Consider Pleur-evac® set to –20 cm H2O
If an adequate and sufficiently current preprocedure CT is available to characterize the collection and suggest an appropriate window, focused diagnostic US examination to confirm the presence of an appropriate sonographic route of access can be performed (Figure 3.8). In fact, for many pleural-based collections the entire procedure can be performed using US guidance, with final confirmation of tube position and function using fluoroscopy (Figure 3.9).
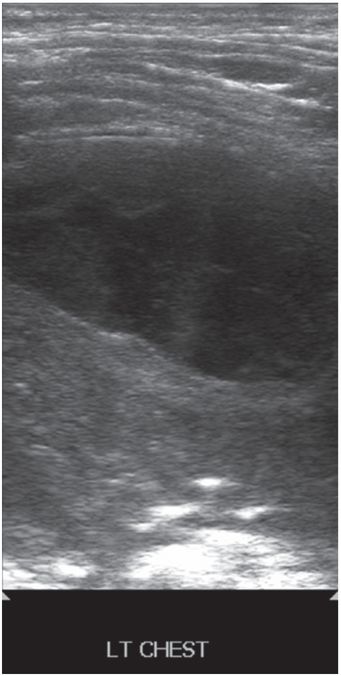
Seven-year-old female with respiratory compromise and pain was found to have a left chest pleural-based elliptical fluid collection as demonstrated on this gray-scale US image using a high-frequency linear transducer.
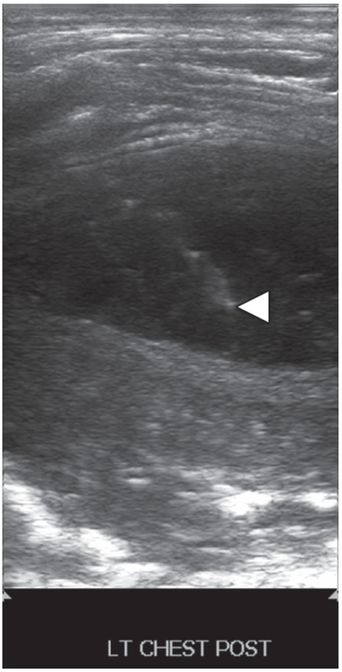
Ultrasound-guided needle access was performed using a 5 French sheathed needle (arrowhead).
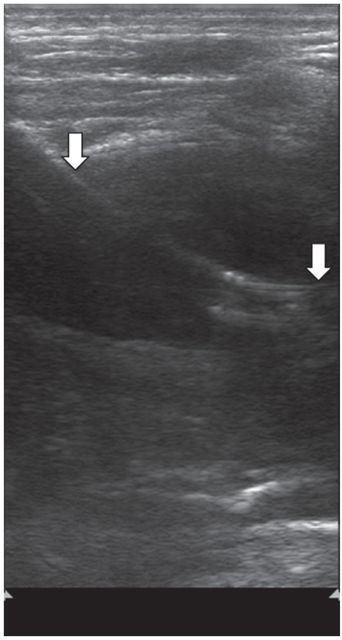
After tract dilation, an 8.5 French pigtail drainage catheter was advanced into the collection with postprocedure US showing catheter position (arrows).
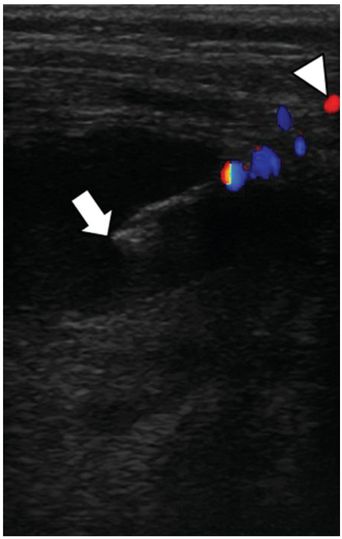
Preprocedure chest CT with vascular contrast in this three-month-old female shows a right-sided light stage 2 parapneumonic effusion with an associated multiloculated abscess (asterisk). Air in the collection suggests the possibility of a spontaneous bronchopleural fistula.
With the patient in a left-side-down decubitus position, a 7 cm 5 French Yueh centesis needle (arrow) was used to access the abscess collection. Color Doppler imaging was used to assist avoidance of the subcostal artery (arrowhead) in the narrow infantile intercostal space.
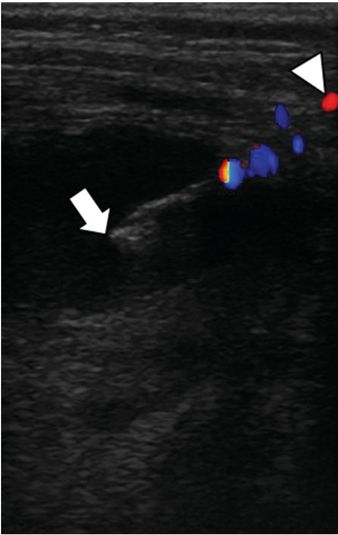
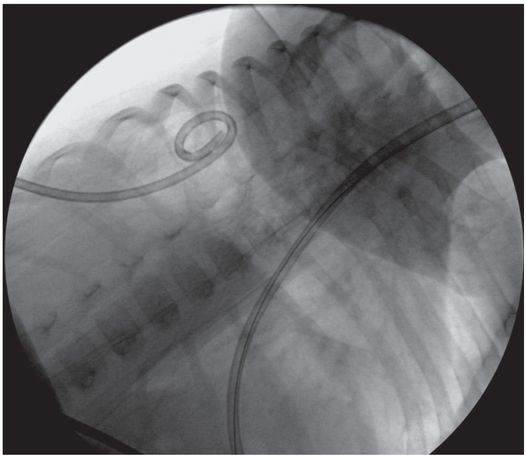
Under continuous US guidance, the stylet was exchanged for a 0.035-inch stiff Amplatz® wire (shown), over which a 7 French Dawson–Mueller pigtail catheter was advanced with its stiffener.
The stiffener and guide wire were removed, and the drainage catheter formed and locked. 50 ml of thick, purulent, blood-tinged fluid was aspirated. Aspiration of air confirmed the bronchopleural fistula. The abscess and effusion resolved without sequelae with tube thoracostomy drainage alone.
Otherwise, CT guidance must be employed. When CT is utilized, either the entire procedure is completed in the CT suite or, if necessary, the guide wire is coiled within the cavity and the patient is transported (while still sedated or anesthetized) to the interventional suite for completion of the procedure using fluoroscopic guidance. In practice, the latter is rarely necessary. Drainage of the abscess can be accomplished using either a Seldinger or trochar technique. Direct puncture with a trochar catheter is seldom used in our practice because there is little room for error in small infants and children, and a misdirected pass may result in injury to adjacent structures. However, this technique may be considered for children with superficial or large collections.
If a Chiba needle is selected for puncture of the abscess cavity (Table 3.9), a 0.018-inch guide wire (e.g., Glidewire®, Newton, Mandril) is inserted and coiled within the cavity. To exchange for a 0.035-inch or 0.038-inch guide wire, a coaxial introducer or micropuncture set can be used. The inner cannula and 0.018-inch guide wire are removed and exchanged for a stiff, non-kinkable 0.035-inch or 0.038-inch (e.g., Glidewire®, Amplatz®, Roadrunner®) guide wire.