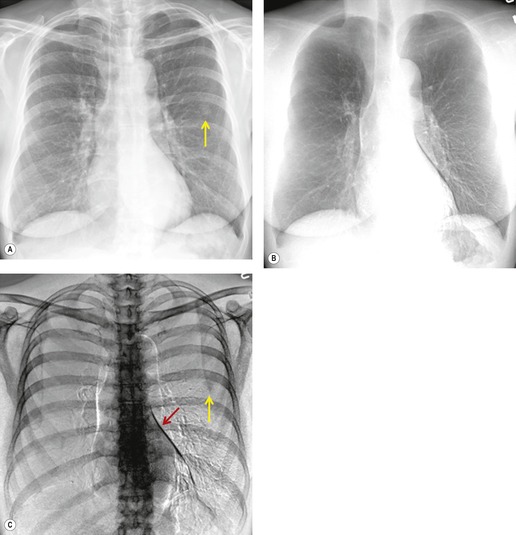
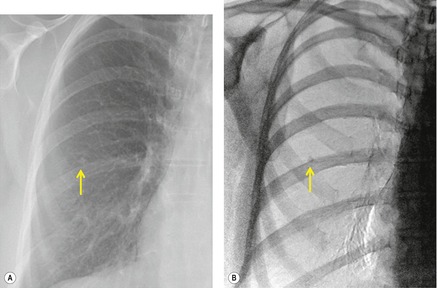
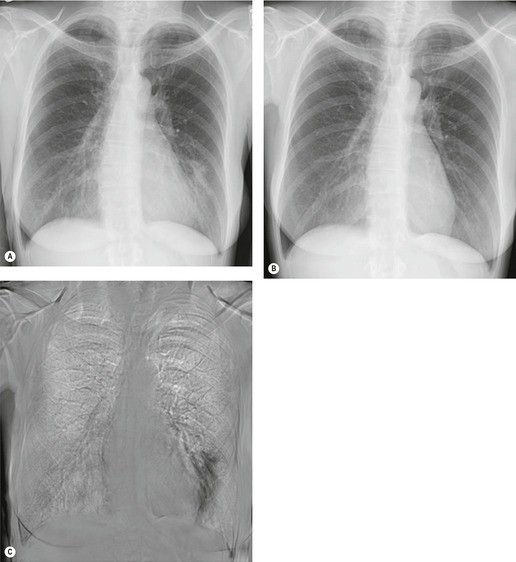
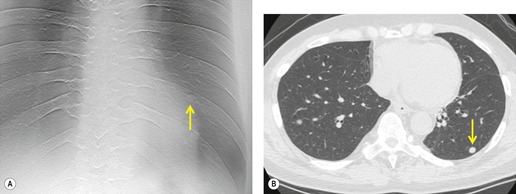
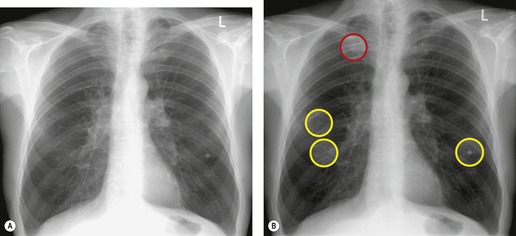
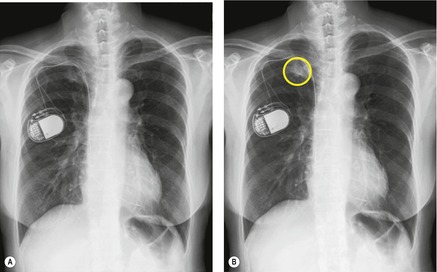
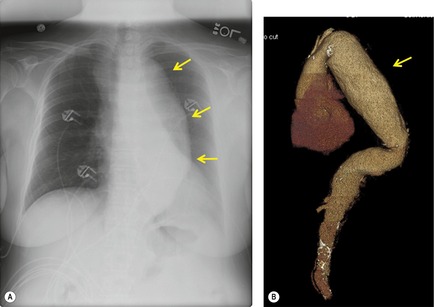
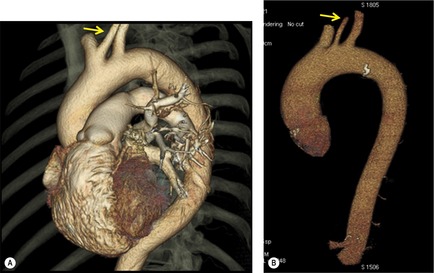
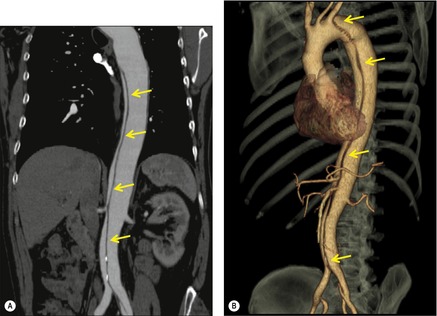
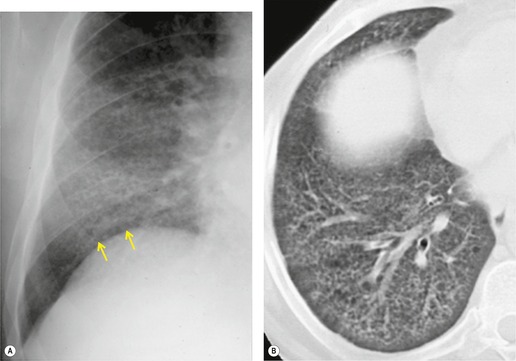
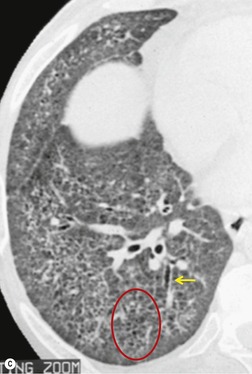
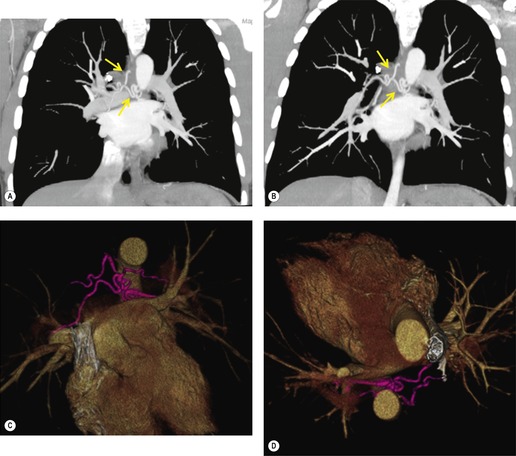
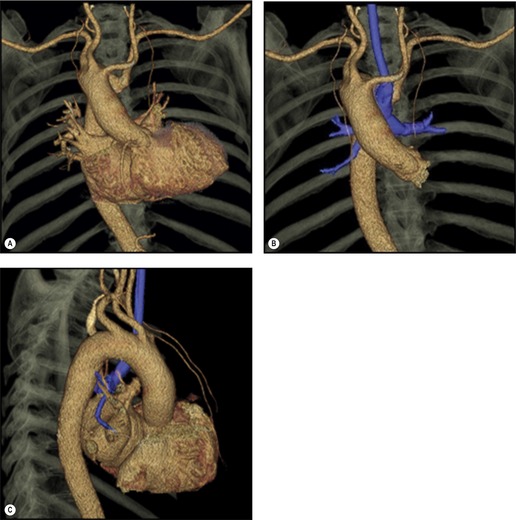
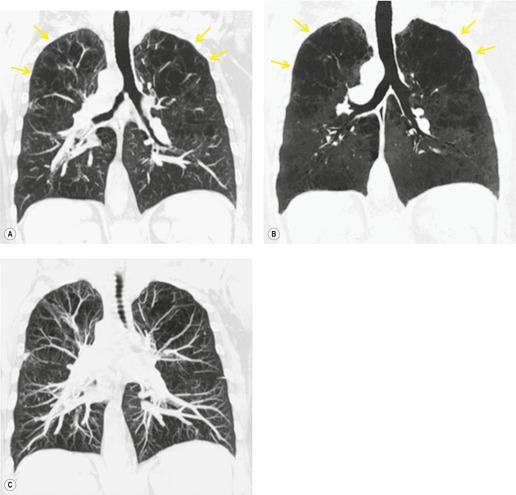
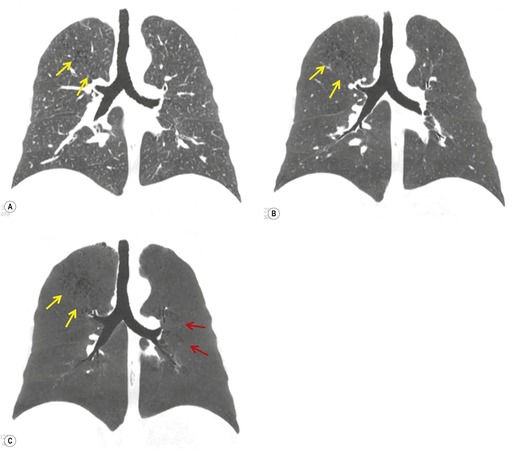
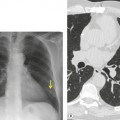
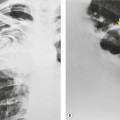
Although a single CT section appears as a two-dimensional image, it has a third dimension of depth. Thus each pixel has a volume, and the three-dimensional element is referred to as a voxel. The average radiographic density of tissue within each voxel is calculated, and the final CT image consists of a representation of the numerous voxels in the section. The single attenuation value of a voxel represents the average of the attenuation values of all the structures within the voxel. The thicker the section, the greater the chance of different structures being included within the voxel and so the greater the averaging that occurs. The most obvious way to reduce this ‘partial volume’ or ‘volume averaging’ effect is to use thinner sections (
Fig. 1.14).
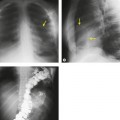
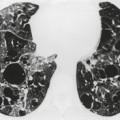
The entire thorax is now usually examined with contiguous sections. MDCT has brought section thickness down to a range of 0.75–2.5 mm. Additional dedicated thin sections are sometimes required to clarify partial volume effects or to study areas of anatomy that are oriented obliquely to the plane of scanning. Specific examples of the use of thin sections to display differential densities, which would otherwise be lost because of the partial volume effect, is the demonstration of small foci of fat within a hamartoma, or of calcifications within a pulmonary nodule. Thin sections of 1–1.5 mm thickness are also used to study the fine morphologic detail of the lung parenchyma (HRCT). Apart from the evaluation of diffuse lung disease, when sampling of a few parts of the lung (traditionally with sections taken at 10–30 mm intervals) is adequate, contiguous section scanning is necessary to allow accurate interpretation in most clinical situations.
A typical pitch of 1 describes the situation, assuming a gantry revolution in 1 second, in which the table travels at 10 mm/s with 10 mm collimation. During a 10-second breathhold, 10 cm in longitudinal axis will be covered. If the travel speed is increased to 20 mm/s, the pitch will be increased to 2 and twice the distance will be covered. In general, the useful range of pitch for thoracic work is between 1 and 2.
74 When detection of small pulmonary nodules is the primary aim, a pitch of less than 1.5 is recommended.
75 Conversely, when radiation dose is a major consideration, scanning at a higher pitch reduces the radiation burden to the patient.
76.77. and 78. Although the spatial resolution of volumetric CT in the transaxial plane is nearly comparable to conventional CT, there is some image degradation because of broadening of the slice profile, inherent in all volumetric CT; this results in additional partial volume averaging in the longitudinal (z-) axis.
79 The faster the table feed, the broader the slice profile will be. The use of a 180° interpolation algorithm produces a slice profile close to the nominal section thickness, although this causes a slight increase in image noise.
69. and 80. Greatly increased z-axis resolution is a feature of MDCT with isotropic imaging (identical resolution in all planes) being the ultimate goal being pursued by manufacturers.
69 This goal has now been reached.
A higher pitch and increased section thickness together enable greater coverage at the expense of increased partial volume effects. However, this can be partly ameliorated by reducing the reconstruction increment, thus producing a larger number of overlapping images.
81. and 82. The ability to retrospectively reconstruct axial images with considerable overlap by choosing a small reconstruction interval is a major advantage of MDCT.
69