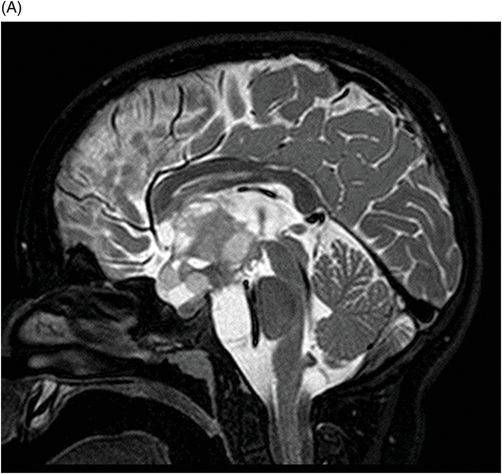
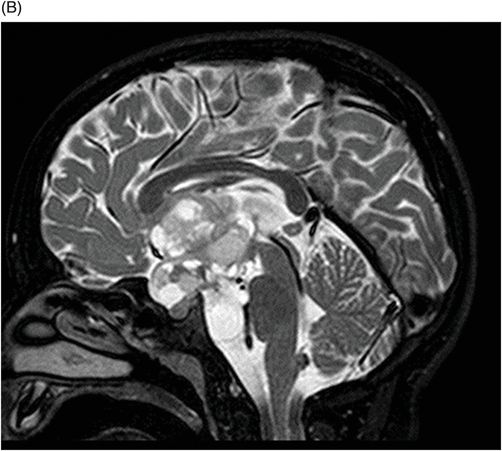
(A–B) Sagittal T2WI through the midline.
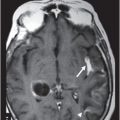
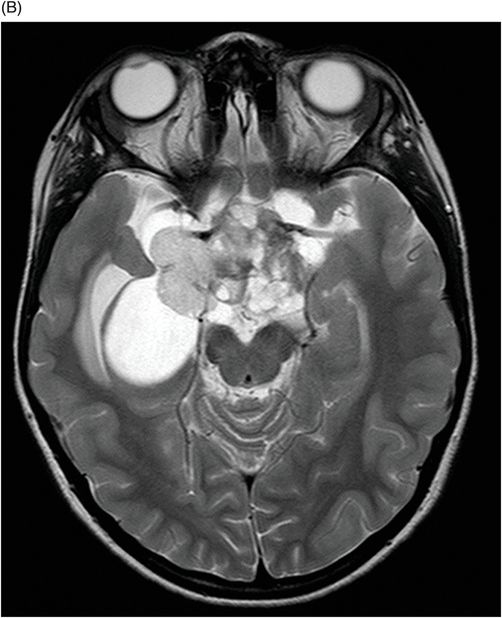
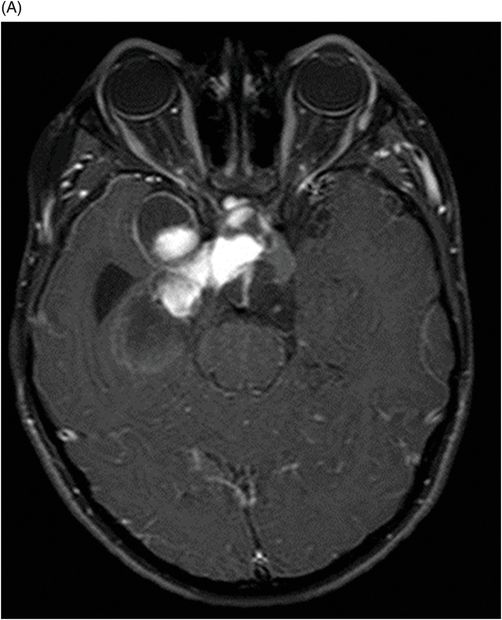
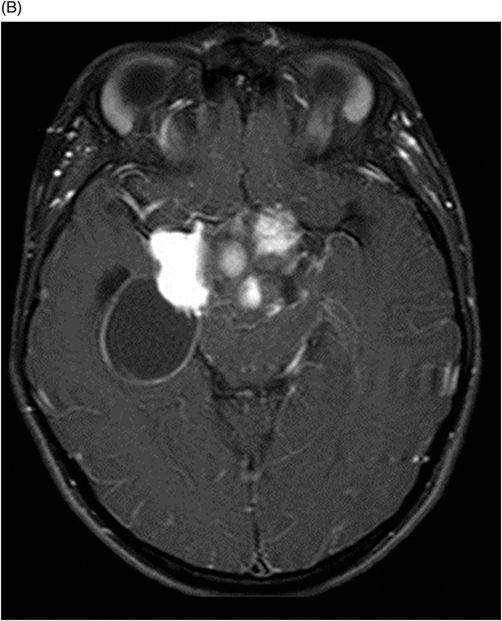
(A–B) Axial T1WI postgadolinium MR image through the suprasellar region.
Pilomyxoid Astrocytoma
Primary Diagnosis
Pilomyxoid astrocytoma
Differential Diagnoses
Juvenile pilocytic astrocytoma
Chordoid glioma of the third ventricle
Ependymoma metastases
Glioblastoma
Imaging Findings
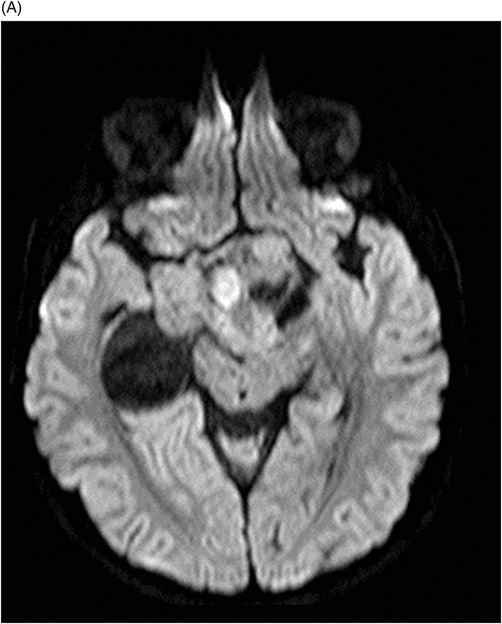
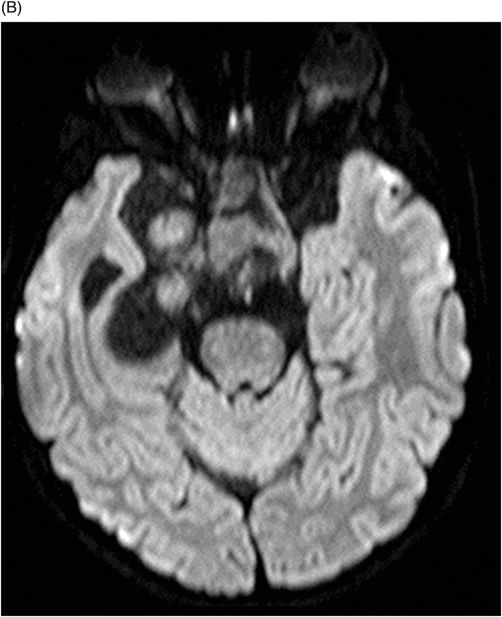
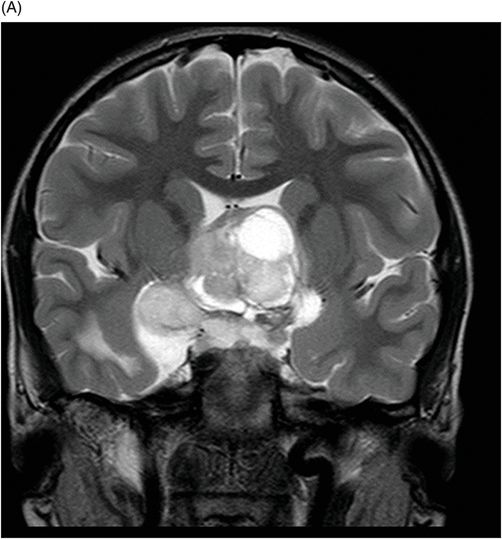
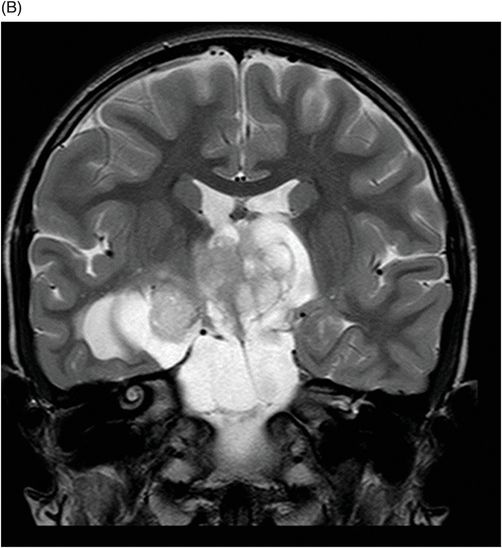
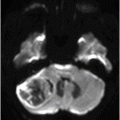
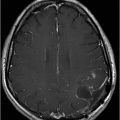
Fig. 115.1: (A–B) Axial T2WI showed a large, lobulated, heterogeneous, solid, cystic mass that predominantly involved the hypothalamus and optic chiasm region, in addition to extending into the right medial temporal lobe. The solid components demonstrated intense contrast enhancement. Fig. 115.2: (A–B) Axial DWI did not demonstrate diffusion restriction. Fig. 115.3: (A–B) Sagittal T2WI demonstrated extension of the lesion’s suprasellar component into the third ventricle. Fig. 115.4: (A–B) Coronal T2WI clearly demonstrated the parasellar component of the lesion, which is larger on the right side. Fig. 115.5: (A–B) Axial T1WI postcontrast showed enhancement of the lesion’s solid component and peripheral enhancement in the cystic component.
Discussion
Chordoid glioma is a rare, slow-growing, non-invasive neoplasm (WHO grade II) containing both glial and chordoid histologic elements, more common in adult patients. It arises from the anterior third ventricle and is frequently adherent to the hypothalamus, ovoid in shape, well circumscribed, located in the anterior third ventricle, and separated from the pituitary gland. They invade the hypothalamus and displace the infundibulum posteriorly. At MR imaging, chordoid glioma appears as a well-defined ovoid mass that is isointense on T1-weighted images and enhances intensely with gadolinium. Most tumors are solid, but in 25% of patients, a small central cystic area is present, but calcification is rare. Ependymoma metastases are exluded in the absence of previous patient history of primary ependymoma. Glioblastoma is a more infiltrative lesion, with necrosis, and is rare in pediatric patients.
In a pediatric patient, a large, predominantly midline, multilobulated, solid-cystic mass centered in the hypothalamus/optic chiasm region, which extends to the medial temporal lobe and demonstrates intense enhancement of the solid component on T2-weighted images, is a classic presentation of a pilomyxoid astrocytoma (PMA).
A recently described neoplasm, PMA was originally classified as an infantile variant of pilocytic astrocytoma (PA). Now recognized as a distinct entity, PMA has a unique histologic appearance and differs from PA in its presentation, as well as its clinical course. A rare childhood tumor, whose incidence is unknown, PMA has been described in patients with a wide age range of presentation from early childhood to late adulthood. However, the most common age of presentation is during early childhood with a mean presentation age of 18 months, lower than that of PA, which is 58 months. There is no known sex predilection for PMA. Like many pediatric tumors, the most common presenting clinical features of PMA are the signs and symptoms related to increased intracranial pressure.
Similar to PA, PMA lesions can occur anywhere along the neuraxis; unlike PA, PMA lesions have a predilection for the hypothalamic/chiasmatic region. Almost 80% of all PMAs are located in this area. Atypical sites of origin such as the cerebellum, thalamus, brainstem, and spinal cord have been described; however, these locations are rare and are usually seen in older children or adult patients. In addition to an older diagnosis-presentation age, PA lesions favor the posterior fossa. Although PMA and PA lesions have many imaging features in common, PMA lesions typically demonstrate a cystic tumor component that is smaller than the solid component, excluding PA as a diagnosis in this patient. Glioblastoma is usually a tumor of adulthood and is extremely rare in the diencephalon region, thus an unlikely diagnosis for this patient.
Typically, PMA is a well-circumscribed tumor with both solid and cystic components. Unlike PA, the cystic component is smaller in comparison to the solid component and a small cyst is present in up to 85% of all PMA tumors. The solid component always demonstrates intense enhancement. Unlike high-grade tumors, the solid component does not demonstrate diffusion restriction or peritumoral edema.
Infiltration is more common in PMA, as compared to PA, as spectroscopic abnormalities have been demonstrated beyond the margin of the tumor. Intratumoral hemorrhage can be seen in up to 20% of PMAs, and if present, can be useful to differentiate PMA from PA, as hemorrhage in PA is rare. Unlike PA, CSF dissemination is more common in PMAs and can be seen on imaging at presentation. Similar to PA, PMA demonstrates a high Cho/Cr ratio, a high lipid peak, and low NAA and peak on MR spectroscopy, a feature of aggressive tumors.
Pilomyxoid astrocytoma is a WHO grade II neoplasm. The dominant histopathologic finding is a myxoid tumor matrix with monomorphic bipolar tumor cells arranged in an angiocentric pattern. Although Rosenthal fibers are typically absent, mitotic figures may be present. Pilomyxoid astrocytoma is associated with shorter progression-free and overall survival rates, with higher recurrence and higher rate of CNS dissemination than other childhood brain tumors. As it is an aggressive tumor, early diagnosis and intervention has the potential to affect short-term patient outcomes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree