TRACHEAL DISORDERS
Tracheal lesions that are part of a generalized pulmonary or systemic disorder, together with congenital, traumatic, and neoplastic processes, are discussed in the relevant chapters. With a few exceptions, consideration is given here only to conditions in adults that are essentially confined to the trachea. Given that the trachea is rightly regarded as something of a blind spot on chest radiography, 1 computed tomography (CT) has become the imaging examination of choice for the investigation of tracheal disorders2.3.4. and 5. (Fig. 12.1), with magnetic resonance imaging (MRI) occasionally providing additional information.6.7. and 8. Coronal reformations and three-dimensional reconstructions of volumetric CT data usefully clarify tracheobronchial anatomy in some situations.9.10.11.12.13.14.15. and 16.
The trachea may be affected by a wide variety of extrinsic or intrinsic processes. Extrinsic processes, particularly masses, displace and distort the trachea, while intrinsic ones cause narrowing, widening, or a mass effect. Tracheal diseases can be further conveniently divided into those that show focal4 and those that show diffuse5 involvement.
Tracheal narrowing
Tracheal narrowing may affect a long or short segment, but since the distinction is somewhat subjective, both types are considered together. The causes of tracheal narrowing, some of which are exceedingly rare, are listed in Box 12.1.
Box 12.1
Extrinsic
Intrinsic
Saber-sheath trachea (Box 12.2)
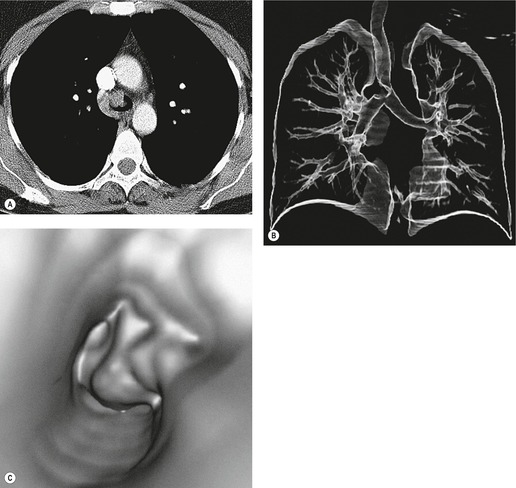
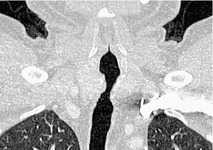
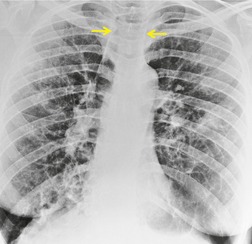
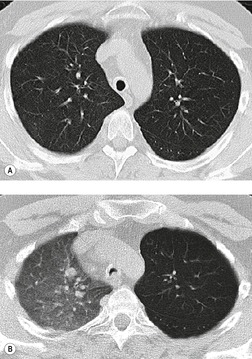
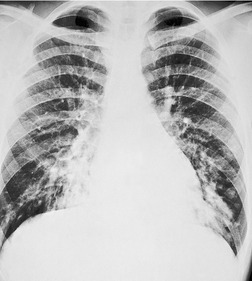
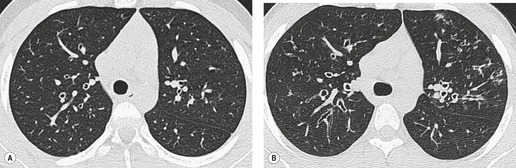
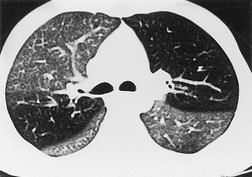
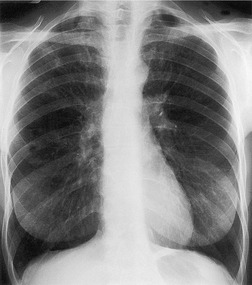
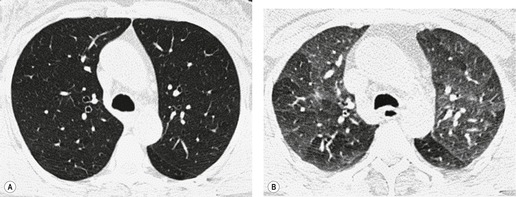
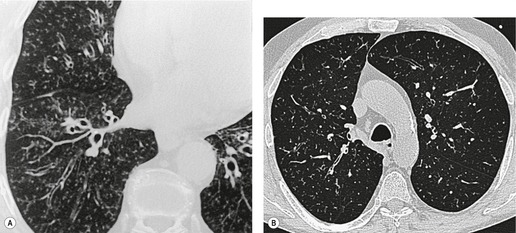
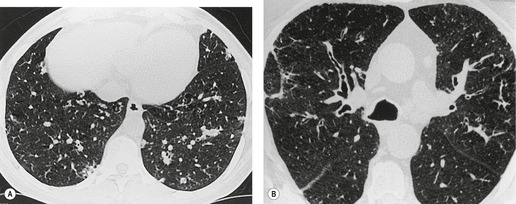
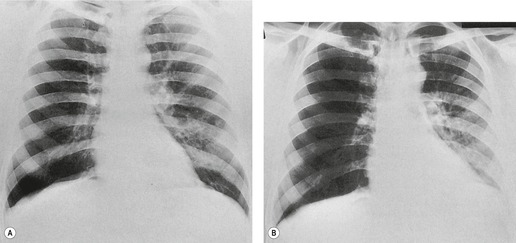
Saber-sheath deformity is limited to the intrathoracic part of the trachea, which is flattened from side to side so that the coronal diameter is two-thirds or less of the sagittal diameter at the same level. 27 The condition occurs almost exclusively in men, usually in those more than 50 years of age; the youngest recorded patient was 37 years of age. 73 Saber-sheath trachea is strongly associated with the presence of chronic obstructive pulmonary disease (COPD), and in one series COPD was present in 93% of patients with the deformity compared with 18% of control subjects. 73 The pathogenesis of the lesion is obscure, but probably it is an acquired deformity related to the abnormal pattern and magnitude of intrathoracic pressure changes in COPD. In a study of 40 patients, 20 of whom had a saber-sheath trachea, there was a significant relationship between the ratio of the coronal:sagittal tracheal diameter on CT and functional residual capacity (r = 0.61, p < 0.001), supporting the view that saber-sheath trachea is a consequence of hyperinflation. 74 A reduction in coronal diameter of the trachea, short of the forme complète of saber-sheath deformity, correlates significantly with forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC). 75
Box 12.2
• Saber-sheath deformity is a common finding in men >50 years and is strongly associated with COPD
• The coronal narrowing of the trachea abruptly returns to normal at the thoracic inlet and carina
• Saber-sheath deformity is usually of no clinical consequence, but can rarely be problematic for patients undergoing intubation
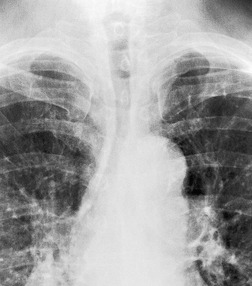
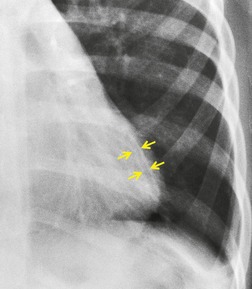
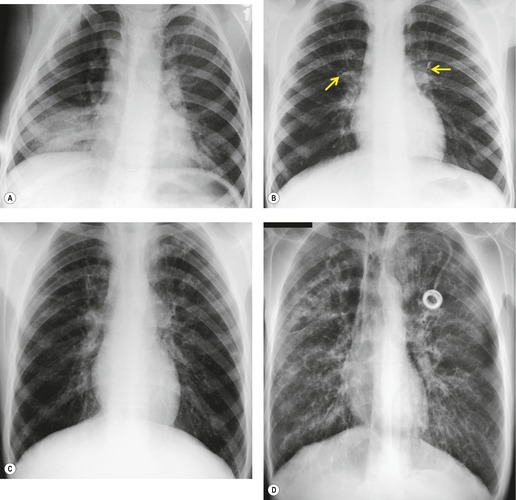
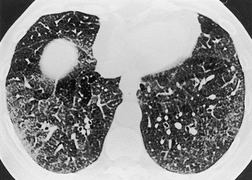
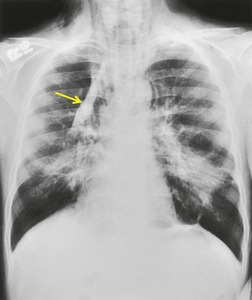
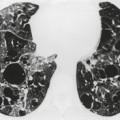
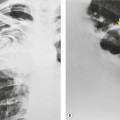
Saber-sheath trachea can be detected on chest radiography (Fig. 12.2) but is better demonstrated on CT. The narrowing usually affects the whole of the intrathoracic trachea, with an abrupt return to normal caliber at the thoracic inlet27 (Fig. 12.3). In Greene’s73 series of 60 patients with saber-sheath trachea and 60 control subjects, the mean coronal diameter of the deformed trachea was reduced to 61% and the sagittal diameter increased to 115%, giving a mean tracheal area of 75% compared with control subjects. Cartilage rings are commonly calcified or ossified (but not thickened) both pathologically and on imaging.27. and 76.
Patients with both saber-sheath trachea and mediastinal lipomatosis are described, in whom the appearance on the plain radiograph therefore strongly resembles a mediastinal mass. 77 The original studies described the trachea as displaying the usual changes in configuration in relation to respiration and considered the trachea to be normally compliant (Fig. 12.4). 73 Others, however, have described an abnormal degree of narrowing on forced expiration76 and have noted that the cross-sectional area was reduced mainly by apposition of the lateral walls with slight invagination of the posterior membrane. 78
Saber-sheath deformity, although usually of little functional consequence, may occasionally cause difficulties for patients undergoing general anesthesia, specifically in terms of intubation,79. and 80. ventilation, 81 and the rare association with negative-pressure pulmonary edema. 82 Preoperative awareness of the deformity is clearly desirable, but even when a chest radiograph is available, a saber-sheath trachea will often be overlooked. In the few individuals in whom marked airflow obstruction can be ascribed to a saber-sheath deformity, intervention in the form of balloon dilation with or without stenting may rarely be required.83.84. and 85.
Cryptogenic stenosis (Box 12.3)
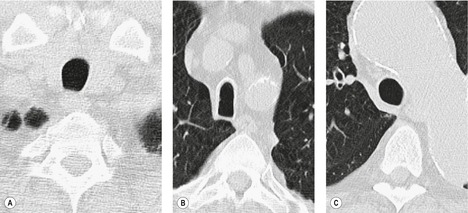
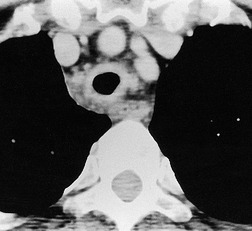
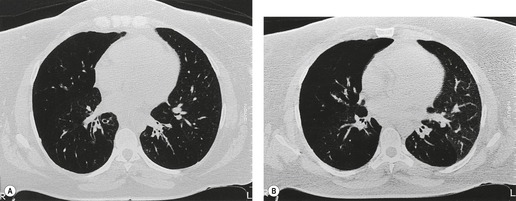
Fibrotic tracheal stenoses of obscure, presumed inflammatory, origin have been described, but truly idiopathic cases are extremely rare. 86 These may occur at any site but tend to be subglottic and are occasionally multiple.45.87. and 88. In a series of 15 cases of idiopathic laryngotracheal stenoses, patients were, in common with other reports, predominantly female (94%) and middle aged (30–60 years). 26 One-third of the stenoses were tracheal, and these were hourglass or eccentric in configuration with smooth or less commonly irregular lobulated internal margins (Fig. 12.5). Stenoses were 2–4 cm long and 3–5 mm wide. The histologic changes were nonspecific, consisting of scarring of the adventitia and lamina propria.
Box 12.3
• Very rare, usually middle-aged women affected
• Eccentric or concentric fibrotic stricture of variable length
• Some cases may be the consequence of an inflammatory vasculitis
Some patients with cryptogenic stenoses have positive titers of antineutrophil cytoplasmic antibodies. 89 In these patients stenoses have been recurrent and the histologic features either granulomatous or nonspecific. It seems likely that such cases are part of the Wegener granulomatosis spectrum.
Tuberculosis (Box 12.4)
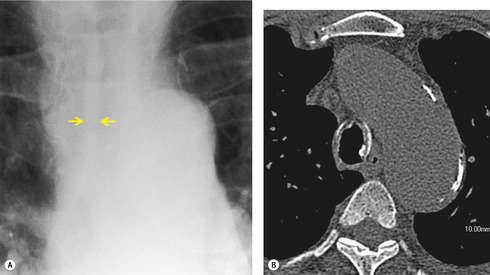
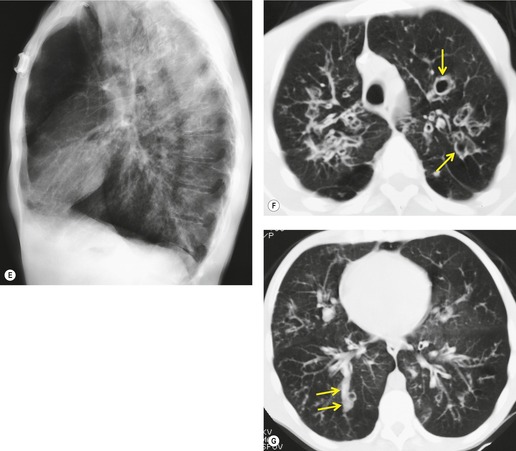
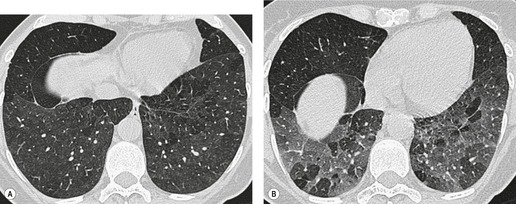
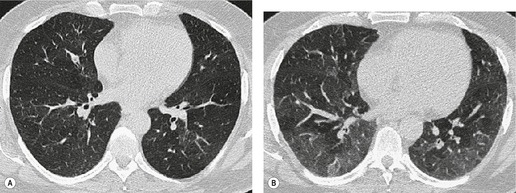
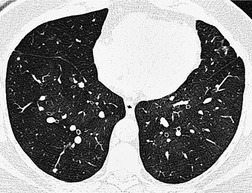
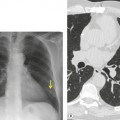
Tuberculosis of the trachea is now very rare. It may be associated with cavitary lung disease and grossly infected sputum.38. and 45. Pathologic study shows mucosal thickening and ulceration and subsequent healing by fibrosis with stricture formation. 45 Occasionally the trachea is involved by direct spread from adjacent nodes24 and fistula formation subsequent to this is described. 90 On CT the extent of irregular and circumferential tracheobronchial narrowing is clearly demonstrated23. and 91. (Fig. 12.6) and in some patients an accompanying mediastinitis (opacification of the mediastinal fat) is evident. CT shows differences between active disease in which the narrowed trachea (and frequently one or other main bronchus) has an irregularly thickened wall; by contrast in the fibrotic or healed phase the trachea is narrowed but has a smooth and sometimes normal thickness wall. 91 Surgical resection or balloon dilation and stenting may be required to treat the resulting stenosis.24. and 92.
Box 12.4
• Tuberculosis confined to tracheal wall involvement is rare
• Often accompanied by involvement of one or other mainstem bronchus
• Pronounced tracheal wall thickening in active disease
• Wall thickness may return to normal in healed phase (lumen narrowed)
Scleroma
Scleroma is a chronic progressive granulomatous infection that affects primarily the nose but may also involve the nasopharynx, larynx, trachea, and bronchi. It is caused by Gram-negative coccobacilli. Scleroma is uncommon in the West, occurring mainly in Asia, north Africa, Central and South America, and eastern Europe. The disease passes through three phases: catarrhal, granulomatous and proliferative, and scarring. 93 Occasionally there is a soft tissue mass or bone destruction suggesting a nasal carcinoma. Laryngeal involvement is usually manifest as glottic or subglottic narrowing and vocal cord thickening.94. and 95. About 5% of patients have tracheal involvement, which is nearly always accompanied by laryngeal disease and almost invariably paranasal sinus disease. 45 Scleroma can be treated by antibiotics such as ciprofloxacin. 41
Tracheo(broncho)pathia osteo(chondro)-plastica (Box 12.5)
Tracheopathia osteoplastica was first described more than 100 years ago, 97 yet it remains a curiosity of obscure etiology. Although rare, it is more common than airway amyloidosis, a condition that some have considered to be the precursor of tracheopathia osteoplastica. 98 Pathologic characteristics include the development of cartilaginous and bony submucosal nodules45 in the trachea and proximal airways. The nodules are typically found in the lower two-thirds of the trachea and in the main, lobar, and segmental bronchi. 99 The disorder, however, sometimes starts more proximally and affects the first tracheal ring region. 65 The osteochondral nodules develop adjacent to the airway cartilages and rarely, 100 if ever, 101 involve the posterior membranous part of the trachea. The nodules give rise to sessile and polypoidal elevations of the mucosa, which produce airway narrowing, 65 and sometimes lobar collapse. 102 The cause of the condition is obscure. One theory considers the nodules to be a form of ecchondrosis of the airway cartilage because of their distribution in the airways and because they have bony, cartilaginous, and fibrous connections to the cartilage rings themselves. 65 A second theory is that the disorder is due to amyloidosis, a condition in which cartilage and bone formation is known to occur. Several workers have in fact found evidence of amyloid in pathologic specimens from patients with tracheopathia.98. and 103. Most patients are male, and the disease usually develops in middle age (sixth decade), although the age range is wide, from 11 to 78 years. 45 The common early symptoms are dyspnea, hoarseness, cough that is often productive, hemoptysis, and recurrent pulmonary infections. 99 The disease progresses very slowly.
Box 12.5
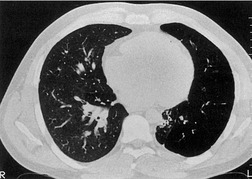
• Very rare slowly progressive disease, characterized by nodular thickening of tracheal cartilage
• Usually involves lower trachea and may extend out as far as segmental bronchi
• Calcification within nodular thickening conspicuous on CT
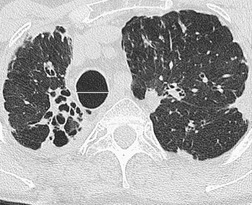
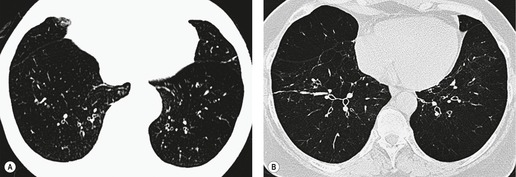
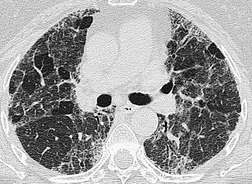
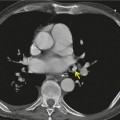
The chest radiograph may be normal99 or may demonstrate evidence of collapse or infective consolidation. Airway calcification has diagnostic value but may be invisible on chest radiography. 45 Tracheal calcification is manifest as irregular or scalloped opacities lying inside the cartilage rings. 65 If the tracheal air column is clearly seen, the irregular nodularity of the tracheal wall and the encroachment of these nodules on the lumen can be appreciated. CT descriptions of the condition are sparse but confirm the expected finding of strikingly nodular thickening of the tracheal wall, with calcification in some of the nodules102.104.105.106. and 107. (Fig. 12.7). The definitive diagnosis is made with bronchoscopy, 66 during which the passage of the instrument may generate a grating sensation.
Tracheal widening
Several studies have documented the normal tracheal dimensions in adults using various imaging techniques.73.108.109.110. and 111. The most useful study using plain chest radiographs is that of Breatnach et al., 108 who studied 808 subjects. Measurements were made 2 cm above the aortic arch and were subject to 8% magnification. Tracheal diameter increased slightly with age, and taking the largest measurements (between 60 and 80 years of age) the coronal diameter in men (mean ± SD) was 19.7 ± 2.2 mm and in women it was 16.8 ± 2.0 mm. In light of these data, a coronal diameter on a chest radiograph of 26 mm in men and 23 mm in women may be considered abnormal. For coronal diameter on CT, this dimension is smaller, since it is not subject to magnification; values are 3–4 mm less.
A limited number of disorders cause tracheal widening, in some the widening is generalized, and in others it is local, as with a tracheocele or following endotracheal tube cuff damage. It is worth pointing out that a few diseases that cause tracheal widening can also be responsible for narrowing the trachea (e.g. relapsing polychondritis). In some forms of diffuse disease, widening is mild and confined to one diameter. Thus in cystic fibrosis the sagittal diameter may be mildly increased (by about 3–5 mm) while the coronal dimension remains normal. 112 However, in a small study that investigated the distensibility and CT dimensions of the trachea in five patients with cystic fibrosis compared with five healthy volunteers, no significant differences were found. 113 In day-to-day clinical practice the most common cause of mild dilatation of the trachea is upper zone or diffuse lung fibrosis as seen in sarcoidosis or tuberculosis114 (Fig. 12.8). Causes of local and generalized tracheal widening are listed in Box 12.6.
Box 12.6
• Tracheobronchomegaly (Mounier–Kuhn syndrome) 115
• Heritable connective tissue disorders
• Cutis laxa118
• Immune deficiency states and recurrent childhood infections
• Ataxia telangiectasia119
• Immunoglobulin deficiency119
• Cystic fibrosis112
• Endotracheal cuff damage
• Relapsing polychondritis72
• Tracheocele120
• Upper lobe fibrosis114
Tracheobronchomegaly (Mounier–Kuhn syndrome) (Box 12.7)
The most striking diffuse increase in tracheal diameter is seen in tracheobronchomegaly (Mounier–Kuhn syndrome). This is a rare abnormality of the trachea and large airways that is associated with recurrent respiratory tract infection and was first described in 1932. 121 The elastic and muscular elements of both the cartilaginous and membranous parts of the trachea are atrophic.115. and 122. In the past the condition was considered to be acquired and to be caused by recurrent infections weakening the airway walls. However, evidence strongly suggests that this is a primary process. 115 Thus in some patients the condition is familial with an autosomal recessive pattern of transmission. 123 There is a recognized association with anatomic variants of the lungs, ribs, and bronchial tree (including a double tracheal lumen, tracheal trifurcation, and a short left main bronchus).115. and 124. Many patients have a history of disease dating back to childhood; 125 presentation as early as 18 months of age has been reported. 126
Box 12.7
• Tracheobronchomegaly is rare and characterized by marked tracheal and bronchial dilatation, usually associated with focal saccular bronchiectasis
• Presents at any age, usually before middle age, with symptoms of bronchiectasis, almost exclusively in males
• May be associated with congenital anomalies
• Prognosis highly variable
No reliable data on prevalence are available, but in a review of 1800 bronchograms the frequency was about 1%. 122 However, it seems likely that the condition is underdiagnosed, since some patients are asymptomatic or have only minor symptoms. 115 It is markedly male predominant (only about 5% of patients are female) and has a possible racial predisposition: in a review of 27 patients 52% were black, 115 indicating a significant excess of black individuals if, as seems likely, most of the populations from which cases were derived were predominantly white. The condition most commonly presents in the third or fourth decade and more than three-quarters of the patients have presented by the age of 40 years. 124 Symptoms, however, often date back a decade or even into childhood.110.122. and 127. Ineffective cough, because of widened central airways, and diverticula of the large bronchi predispose the patient to recurrent pulmonary infections. A minority of patients have no or surprisingly mild symptoms.115. and 128. Pulmonary function tests usually show airflow obstruction, and increased total lung capacity and residual volume, but occasionally results are normal.115. and 129. Airway compliance is increased, and dynamic airway collapse on expiration and cough can be demonstrated.110.115. and 130. The prognosis is variable; some patients have recurrent infections leading to progressive lung damage, bronchiectasis, scarring, and eventual respiratory failure, whereas others survive into old age with relatively mild, nonprogressive disease. 115
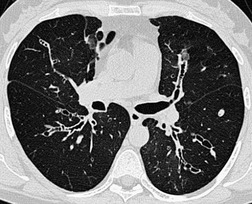
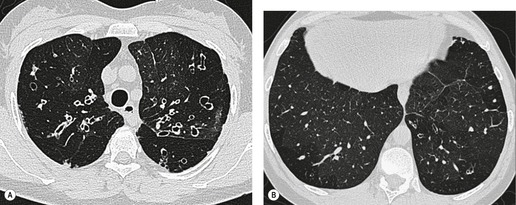
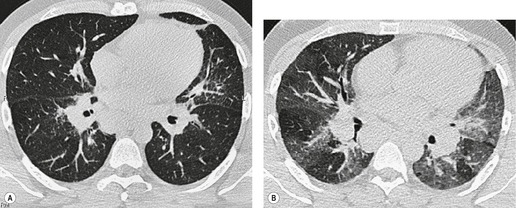
The diagnosis is based on imaging findings. The immediately subglottic trachea has a normal diameter, but it expands as it passes to the carina (Figs 12.9 and 12.10) and this dilatation usually continues into the major bronchi.122. and 124. The dilatation may vary from segment to segment but overall tends to be relatively even. Atrophic mucosa prolapses between cartilage rings and gives the trachea a characteristically corrugated outline that on a plain radiograph is best appreciated on the lateral view.123. and 131. Corrugations may become exaggerated to form sacculations or diverticula.124. and 132. Tracheal changes are well shown on CT78.128.132.133. and 134. (Fig. 12.11). Large vessels may indent the overcompliant trachea. 132
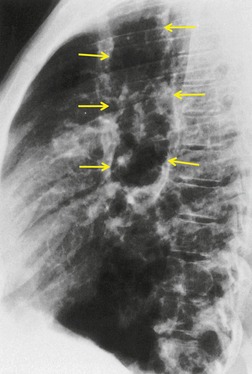 |
| Fig. 12.10 Tracheobronchomegaly. A lateral view of the trachea of the same patient as in Figure 12.9 shows a grossly dilated trachea (arrows). |
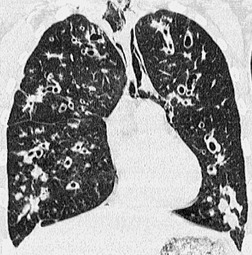
The bronchiectasis of tracheobronchomegaly particularly affects first- to fourth-order branches123. and 133. (Fig. 12.12). Characteristically there is an abrupt transition from large central airways to normal peripheral ones.122. and 132. Small branches that arise from bronchiectatic segments tend to remain patent; this is atypical of bronchiectasis in general, in which they are usually obliterated. The dramatic but delicate nature of the typical saccular and cystic bronchiectasis is well shown on high-resolution CT (HRCT) (Figs 12.11 and 12.13). Dynamic collapse of the trachea and central airways on expiration and cough can be demonstrated by a variety of imaging techniques, including CT.130.132.135. and 136.
Dilatation of the trachea is generalized in tracheobronchomegaly, whereas in other conditions the trachea is usually locally dilated. For example a tracheocele is defined as a localized ballooning of the membranous posterior tracheal wall of obscure etiology.137. and 138. Tracheoceles tend to arise from the right posterior tracheal wall, and their size varies with transmural tracheal pressure. 131 They may affect the cervical137 or thoracic trachea and in the latter instance may be seen as a paratracheal, thin-walled cyst with or without an air–fluid level. 120 Smaller, frequently multiple outpouchings (diverticula) may arise from the trachea and mainstem airways. 139 Small diverticula are a feature of tracheobronchomegaly140 and may reflect cystic dilatation of mucous gland ducts, 131 perhaps similar to those seen in chronic bronchitis (see Fig. 12.70, p. 756).
Tracheomalacia (Boxes 12.8 and 12.9)
Tracheomalacia is present when tracheal compliance is increased because of ‘softening’ of the tracheal wall. It is usually a localized rather than a generalized process and may be congenital or acquired.141. and 142. Important causes of tracheomalacia are listed in Box 12.8. The increase in compliance is due to the loss of integrity of the wall’s structural components and is particularly associated with damage to the cartilage rings.
Box 12.8
Box 12.9
• There are many causes of weakening and hypercompliance of the tracheal wall (tracheomalacia)
• Because of diagnostic uncertainty the incidence of tracheomalacia is unknown
• Standard inspiratory CT is grossly insensitive for the detection of tracheomalacia
• A lunate configuration of the trachea on expiratory CT may reflect a degree of tracheomalacia but there are no exact criteria for making the diagnosis
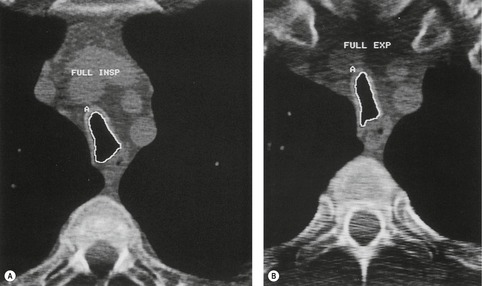
Tracheomalacia is characterized by abnormal collapse of the trachea during respiration. 161 The pressure outside the intrathoracic trachea approximates to pleural pressure and outside the cervical trachea it is atmospheric. When the glottis is open, the luminal pressure is atmospheric. Thus imaging the intrathoracic trachea at full inspiration and expiration permits observation of the effect of a modest change in transmural pressure (~25 cm of water). During dynamic maneuvers such as cough, forced expiration, and forced inspiration the luminal pressure of the intrathoracic trachea is modestly increased or decreased. At the same time large excursions in pleural pressure are generated, and with suitable imaging intrathoracic tracheal compliance can be assessed under these more stressful conditions than are produced by static maneuvers.162. and 163.
Until recently the most common cause of tracheomalacia reported in adults was damage following placement of tracheostomy and endotracheal tubes. However, since the introduction of wide, low-pressure cuffs the problem has largely disappeared. Tracheostomy-related hypercompliant segments may develop at the site of the stoma, at the level of the cuff, or in between; in the last instance the pathogenesis is thought to be damage caused by infection associated with stagnant secretions. 151 An overcompliant tracheal segment may or may not be accompanied by a stenosis. 164 An endotracheal tube cuff lying in a hypercompliant segment appears overinflated, and this may provide an early clue to the presence of tracheomalacia. 152
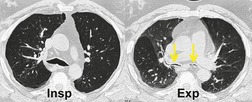
Normally the intrathoracic trachea is round in cross-section or slightly oval with coronal diameters less than sagittal. Occasionally, however, coronal diameters are markedly larger than sagittal ones, producing a lunate configuration (coronal:sagittal ratio >1) to the trachea. A lunate trachea is easily deformed in a sagittal direction, giving rise to a particular form of tracheomalacia. A lunate trachea may be associated with COPD, which is probably the commonest cause of tracheomalacia, 141 but in others it is cryptogenic. Importantly, in one series of 17 patients with bronchoscopically confirmed tracheomalacia, only one individual (6%) had a lunate-shaped trachea on inspiratory CT, compared with nine (53%) individuals who showed an exaggerated lunate or crescentic (‘frown’) shape on dynamic expiratory CT, 165 highlighting the insensitivity of static inspiratory CT for the detection of tracheomalacia.
During forced expiration or coughing a normal trachea shows narrowing both coronally and sagittally, the latter caused by invagination of the membranous posterior wall. Reliable data on the degree of narrowing to be expected are not available, and most studies have been performed in ignorance of the transmural pressure gradient generated. Some authors consider that caliber changes of more than 50% indicate increased wall compliance.157. and 166. Changes in cross-sectional area, and sagittal and coronal diameters in patients with acquired tracheomalacia have been studied on static inspiratory and expiratory CT by Aquino et al. 167 Patients with tracheomalacia showed considerably greater diminution in sagittal (not coronal) diameter and cross-sectional area (Fig. 12.14); more specifically, there was a greater than 89% chance of tracheomalacia if the tracheal cross-sectional change was more than 18% (upper trachea) or 28% (mid-trachea), particularly if the sagittal diameter decreased by more than 28%. 167
With the advent of rapid acquisition CT it has become possible to image the trachea during quiet breathing168 and forced dynamic maneuvers.135.163. and 169. During forced inspiration and expiration the intrathoracic tracheal cross-sectional area varies by 35% ± 18% (SD) 169 and, in view of these data, Stern and co-workers169 suggested that changes in area greater than 70% indicate tracheomalacia (Fig. 12.15), a figure recently endorsed by Boiselle et al. 170 Dynamic studies with CT are particularly effective in identifying tracheomalacic segments.135.163.168.169. and 170. It is unsurprising that the degree of tracheal collapse considered abnormal varies widely depending on the nature and vigor of the expiratory maneuver (breathholding at end expiration167 through to forced coughing163) and data on the normal range of tracheal wall excursion in a large population of healthy individuals are still needed.
The exact incidence of individuals with clinically important tracheomalacia, of whatever cause, is not easy to divine. In a review of patients undergoing CT pulmonary angiography for suspected pulmonary embolism, careful scrutiny of the major airways revealed that 10% of these patients had possible tracheomalacia (trachea showing luminal narrowing of 50% or more; as noted above this criterion may result in overcall). 166 An important message of this study is that abnormal narrowing or collapse of the major airways may be easily overlooked, particularly when the CT examination of the dyspneic patient is targeted at other possible causes, such as pulmonary embolism, and so does not include expiratory maneuvers to allow formal assessment of major airway collapsibility.
In infants and children some cases occur secondary to external compression of the trachea (aberrant subclavian arteries, vascular rings, etc). 171Primary tracheomalacia, caused by defects or complete deficiency of the supporting cartilaginous structures, is rare146. and 172. and may be associated with more severe congenital malformations such as esophageal atresia.147.173. and 174. The cartilage is deficient and the membranous portion of the trachea is widened. 175 The entire airway, or just short segments, may be affected. Affected infants may not manifest symptoms for several months after birth. 176 Many cases of tracheomalacia, particularly milder forms, are managed conservatively because spontaneous resolution in the first few years of life is common. 161 In such self-limiting cases the presumed defect is immaturity of cartilage. 142 The diagnosis is made by observing marked expiratory collapse of the trachea in the anteroposterior dimension. 176 Bronchoscopy is probably the mainstay for diagnosis of tracheomalacia, 143 but bronchoscopy in neonates usually requires general anesthesia and is thus a last resort. Less invasive approaches to diagnosis include fluoroscopy, dynamic helical or electron-beam CT,9.135. and 176. and MRI.148. and 177.
Tracheal filling defects
In adults, tracheal filling defects are most commonly produced by neoplasms (see Chapter 13), but there are a number of other causes (Box 12.10).
Box 12.10
Neoplasm53. and 178.
• Benign (epithelial and mesenchymal)
• Malignant
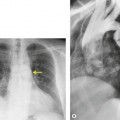
• Carcinoma (squamous, adenoid cystic [Fig. 12.1], adenocarcinoma)
• Sarcoma
• Plasmacytoma
• Lymphoma
• Malignant invasion from without
Infection or granulomatous
• Viral papilloma
• Membranous croup
• Fungal infection
• Tuberculosis
• Rhinoscleroma
• Wegener granulomatosis
• Bacillary angiomatosis
Trauma
• Hematoma
Miscellaneous179
• Ectopic thyroid and thymus
• Amyloidosis
• Tracheopathia osteoplastica
• Foreign body
• Mucoid pseudotumor
• Cyst or mucocele
Ectopic thyroid
Ectopic thyroid is a rare cause of an intratracheal filling defect, 180 with about 150 cases in the literature. 181 Females outnumber males by about 3 : 1. 182 The ectopic thyroid may be histologically normal, although it is usually goitrous. Occasionally it is malignant. 183 Three-quarters of intratracheal thyroid nodules are associated with extratracheal goiter. Foci of ectopic thyroid may occur anywhere between the subglottis and main carina, 181 but typically they are a few centimeters below the vocal cords, arising as smooth, sessile nodules from the posterolateral wall of the trachea. 182 Ectopic thymus has also been described as causing a submucosal intratracheal mass. 184
Tracheal papilloma
Squamous papillomas of the trachea are usually multiple and may be a manifestation of laryngeal papillomatosis with tracheobronchial dissemination (see p. 834).183. and 185. Recurrent respiratory papillomatosis seems to have a bimodal age distribution with the mean age of the juvenile form being 2 years old, and adult-onset approximately 40 years of age.37. and 186. Solitary squamous cell papillomas of the trachea are also recognized in adults and may undergo malignant transformation.183. and 187.
Paratracheal cysts
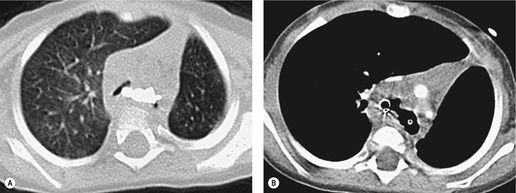
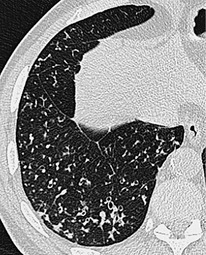
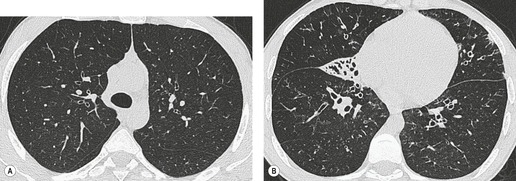
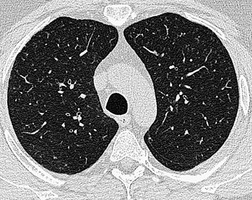
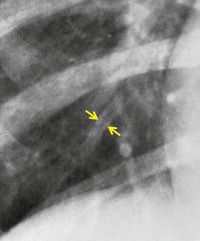
Small air-filled cysts (ranging in size from 1 cm to a few centimeters) arising from the mid- or upper trachea are usually an incidental radiographic or CT finding188 (Fig. 12.16). They usually occur on the right side, and on thin-section CT a communicating channel between the cyst and trachea may occasionally be identified. Although usually considered rare, a Korean study has characterized right paratracheal cysts in 65 individuals. 189 In all but one patient the cysts arose from the right posterolateral aspect of the trachea. Most cysts were approximately 1 cm in diameter and the majority of patients had functional evidence of obstructive lung disease. 189 Nevertheless, it is not entirely clear whether these cysts are congenital or acquired, 188 and their relationship, in terms of pathogenesis, to tracheoceles120 is unclear. Surgical resection may be undertaken because of the potential for infection within the cyst.
Tracheoesophageal fistula
In the pediatric age group tracheoesophageal fistula is, almost invariably, congenital. 190 Occasionally such congenital fistulas present in adulthood. 191 Malignant neoplasia, particularly esophageal, is the most common cause in adults. Infection and trauma are the most frequent nonmalignant causes (Fig. 12.17). The etiology of bronchoesophageal fistula is similar to that of tracheoesophageal fistula and various causes are shown in Box 12.11.
BRONCHIECTASIS
Bronchiectasis is a chronic condition characterized by irreversible dilatation of bronchi, caused by inflammation. 203 The qualification ‘irreversible’ is included in the definition to exclude the transient airway dilatation that has been observed in pneumonia and atelectasis.204.205. and 206. Dilatation of the airway in these circumstances is probably partly related to inflammatory changes in bronchial walls, altering compliance, and to exaggerated lung stresses after collapse. On rare occasions some adult patients show some reversibility of what would otherwise be considered typical idiopathic bronchiectasis. 207 By contrast, in children reversibility of what may be considered bronchiectatic airways by HRCT criteria is more frequent. 208
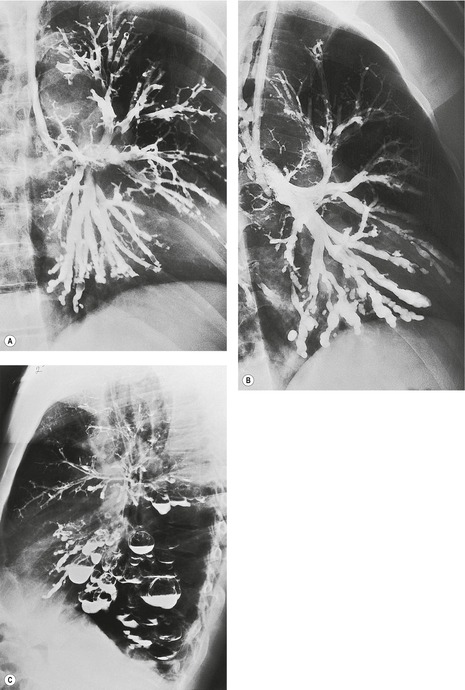
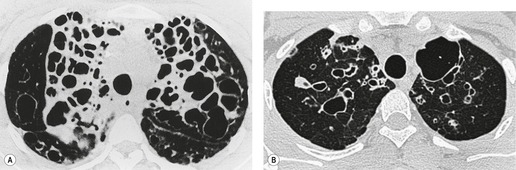
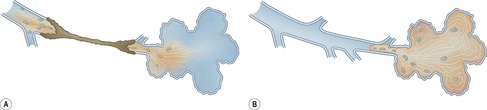
On macroscopic study, bronchiectatic airways are dilated in a variety of patterns that were historically classified into two203 or three types. 209 The three-grade Reid classification is applicable to gross pathologic, bronchographic, and CT appearances. However, the clinical utility of designating bronchiectasis as cylindrical, varicose, or cystic is not obvious, although it is generally agreed that cystic bronchiectasis represents the most advanced disease. The Reid classification, most elegantly shown by bronchography (Fig. 12.18), is as follows:
• Cylindrical bronchiectasis. Bronchial dilatation is mild, and the bronchi retain their regular and relatively straight outline.
• Varicose bronchiectasis. Bronchial dilatation is greater than in cylindrical bronchiectasis and is accompanied by local constrictions that give the airway an irregular outline. Obstruction and obliteration of small airways is more pronounced.
• Cystic (saccular) bronchiectasis. This is the most severe form of bronchiectasis. The airway takes on a ballooned appearance and the number of bronchial divisions is greatly reduced.
Histologic study of bronchiectatic airways shows the walls to be thickened and chronically inflamed with chronic granulation tissue and the bronchial arteries to be hypertrophied210. and 211. (and these bronchial arteries may be conspicuous at CT). 212 Ciliated epithelium is largely replaced by squamous epithelium or areas of squamous metaplasia. The mucosa is sometimes ulcerated or thrown into transverse ridges by circular muscle hypertrophy. Airways are surrounded by fibrosis and there may be organizing pneumonia in the adjacent parenchyma. 211
The pathogenesis of bronchiectasis is complex, as shown by the large number of recognized causes and associations.210.213.214. and 215. The most important and commonly implicated pathogenic factor is bronchial wall weakness, often brought about by infective inflammatory damage. It seems probable that such damage is self-perpetuating and this has led to the ‘vicious circle’ hypothesis, which proposes that colonizing microbes impair normal host clearance mechanisms, thereby modifying the environment within the airways so allowing microbial growth. The host immune response is ineffective in dealing with the microbial colonization and, paradoxically, the immune response further damages mucociliary clearance, thus setting up a vicious circle of host damage and further microbial growth. 216 Recognized associations and causes210. and 214. are listed in Box 12.12.
Box 12.12
Postinfection
Obstruction
Inhalation and aspiration
Impaired host defense and immunologic
Gross bronchiectasis is characterized by persistent cough, with copious purulent sputum and recurrent pulmonary infections. Symptoms frequently date from childhood, when there may have been a precipitating pneumonic event. With widespread disease there may be dyspnea and, ultimately, cor pulmonale. Such severe bronchiectasis is becoming unusual and is largely limited to patients with impaired defense mechanisms. A recognized presentation of mild bronchiectasis is recurrent hemoptysis, 276 but mild bronchiectasis may be asymptomatic.
The classic description of the radiographic changes in bronchiectasis is that of Gudbjerg. 277 In this historic series 93% of radiographs were said to be abnormal. Although this might seem a high figure as judged by some clinical reports,278. and 279. later studies have reiterated that careful analysis of chest radiographs of patients with bronchiectasis will reveal abnormalities in the majority of cases.280. and 281. Some varieties of bronchiectasis such as occur in cystic fibrosis and the ciliary dyskinesia syndrome282 almost invariably show radiographic changes, which comprise:
• Visible bronchial walls – these are visible either as single thin lines or as parallel line opacities (Fig. 12.19).
• Ring and curvilinear opacities – these are generated by thickened airway walls seen end on. Ring opacities range in size from 5 mm to 20 mm and can have very thin (hairline) walls (Figs 12.20 and 12.21). They may contain air–fluid levels.
• Plugged airways – these give rise to band shadows of variable size (Fig. 12.22), which may branch, giving V, Y, or even more complex-shaped opacities.
• Vascular structures – these may appear increased in size and may be indistinct because of adjacent peribronchial inflammation and fibrosis.
• Volume change – in generalized bronchiectasis, such as that associated with cystic fibrosis, there is often generalized overinflation282 (Fig. 12.20). Localized forms, however, are frequently accompanied by atelectasis (Fig. 12.22), which may be mild and detected only because of vascular crowding, fissural displacement, or obscuration of part of the diaphragm (Fig. 12.19).
• Other signs – these include evidence of scarring, bulla formation, and pleural thickening. Areas of pulmonary consolidation may be due to infection, for example Pseudomonas aeruginosa, but can also be a manifestation of allergic bronchopulmonary aspergillosis, which may be superimposed on other bronchiectatic conditions (for example, cystic fibrosis). 283
HRCT signs of bronchiectasis
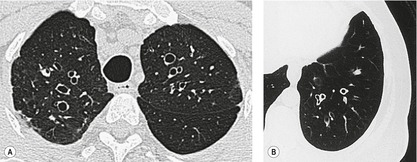
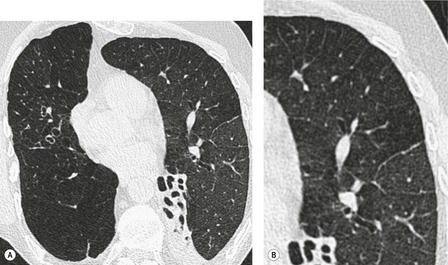
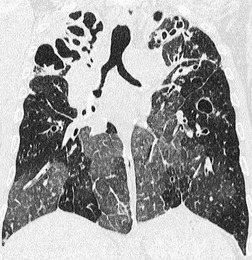
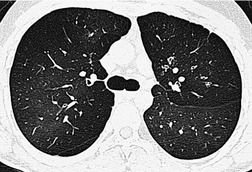
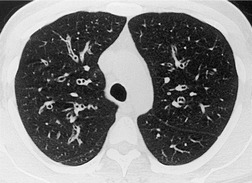
By pathologic definition, dilatation of the airways is a prerequisite for the diagnosis of bronchiectasis. Thus the major sign of HRCT bronchiectasis is dilatation of the bronchi (with or without bronchial wall thickening). The characteristics of bronchiectasis on CT, first described over 25 years ago, 284 have withstood the test of time with subsequent minor refinements and additions.285.286.287.288.289. and 290. Absolute measurements for the diameter of all generations of the bronchi are not available for normal individuals, and relating the size of a bronchus to its immediately adjacent (homologous) pulmonary artery has been the most widely used criterion for the detection of abnormal dilatation. 291 In normal individuals the overall diameter of a bronchus is approximately the same, at any given level, as that of its accompanying pulmonary artery. The ratio of the diameter of the bronchus (internal lumen) to the pulmonary artery diameter for subjects has been estimated to be 0.62 ± 0.13 (mean ± SD). 292 Recognition of abnormal dilatation of a bronchus by comparison with its accompanying pulmonary artery can be readily made for airways running perpendicular to the CT section. When bronchial dilatation is marked, the cross-sectional appearance of the combined bronchus and artery resembles a pearl or signet ring293 (Fig. 12.23).
In healthy individuals minor discrepancies in the bronchoarterial diameter ratio may occasionally be encountered.291. and 294. Furthermore, there are many factors that can cause transient or permanent changes in diameter of the relatively compliant pulmonary arteries, so invalidating this sign. For example, a left-to-right cardiac shunt will result in generalized increase in perfusion and thus caliber of the pulmonary arteries; conversely any cause of underventilation of a region of lung will result in hypoxic vasoconstriction. Thus bronchial dilatation in isolation cannot always be regarded as diagnostic of bronchiectasis and this is especially true in children. 208 This caveat has been emphasized by Lynch et al., 294 who showed that 59% of normal individuals had at least one bronchus with a diameter greater than that of the homologous pulmonary artery; in these healthy subjects bronchial wall thickening was not a common association. One of the factors that appears to have contributed to the apparently high frequency of a decreased bronchoarterial ratio in normal individuals, in this particular study, was hypoxic vasoconstriction: the study group investigated were all examined in Colorado, at approximately 1600 m above sea level. 294 Kim et al. 292 subsequently performed a detailed study which compared the bronchoarterial ratio of normal individuals at sea level with that of normal individuals at high altitude (1600 m above sea level). The mean bronchoarterial ratio in individuals at altitude was 0.76 (SD 0.14) compared with 0.62 (SD 0.13) at sea level (p < 0.001).
When airways lie parallel to the plane of section, abnormal dilatation is recognized by a lack of normal tapering, producing a tramline or even flared appearance (Fig. 12.24). These airways are conspicuous in the lung periphery because of associated bronchial wall thickening.
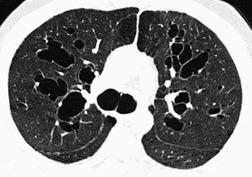
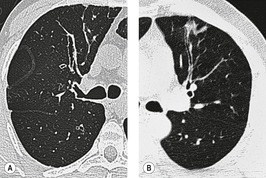
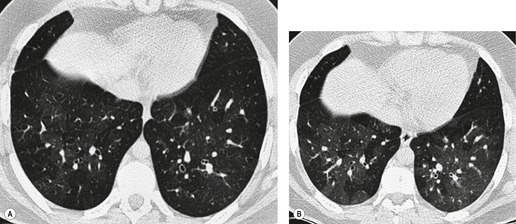
The cylindrical and varicose patterns of bronchiectasis, described by Reid, 209 can be appreciated only for bronchi that lie within the plane of the CT section. Cylindrical bronchiectasis is by far the commonest morphologic pattern of bronchiectasis identified on CT. 295 A bronchoarterial ratio of greater than 1 has been reported in 95% of patients with cylindrical bronchiectasis. 290 Varicose bronchiectasis is characterized by a beaded appearance (Fig. 12.25) and cystic (or saccular) bronchiectasis is seen as thin-walled cystic spaces which may contain fluid levels. In cystic bronchiectasis, recognition that large cystic airspaces represent massively dilated bronchi may sometimes be difficult or impossible (Fig. 12.26). In this type of severe bronchiectasis the accompanying pulmonary artery is often obliterated. When varicose bronchiectatic airways are imaged in cross-section, they may appear as either cystic or cylindrical bronchiectatic airways because the characteristic corrugation of the bronchial walls cannot be identified in this orientation. Sections obtained at end-expiration have been advocated to differentiate cystic bronchiectasis from other cystic lung diseases; 296 bronchiectatic airways usually decrease in size on expiratory scans in contrast with other cystic lesions. However, one study has reported that most cystic lesions in the lungs, whether bronchiectatic or another etiology, decrease in size, 297 rendering this sign of doubtful discriminatory value.
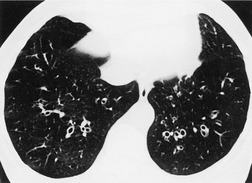
Bronchial wall thickening is a usual, but inconstant, feature of bronchiectasis (Fig. 12.27). Problems with this variable feature have been widely debated and the definition of what constitutes ‘abnormal bronchial wall thickening’ remains unresolved. 289 Minor to mild degrees of bronchial wall thickening are seen in healthy subjects, particularly elderly individuals, 298 asthmatic people, individuals with acute lower respiratory tract infections, 299 and asymptomatic smokers.294.300. and 301. There is no simple and robust criterion for the identification of abnormal bronchial wall thickening. 302 Remy-Jardin et al. 300 defined a bronchus as being thick walled when the bronchial wall was at least double the thickness of a normal bronchus. However, such a judgment is possible only when comparable ‘normal’ bronchi can be identified. An assessment can also be made by relating the bronchial wall thickness to the diameter of accompanying pulmonary artery,295. and 303. but in practice this does not overcome the difficulties of identifying subtle bronchial wall thickening. 304 Diederich et al. 305 defined abnormal bronchial wall thickening as being present if the internal diameter of the bronchus was less than 80% of the external diameter. While this sign was associated with good interobserver agreement it cannot be applied to conditions in which there is marked accompanying bronchial dilatation.
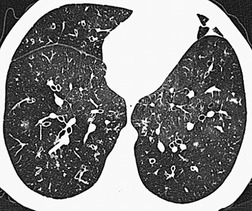
Normal airways within 2 cm of the visceral pleural surface are not usually visible because their walls are below the spatial resolution of HRCT. 306 However, perhaps as a consequence of improving CT technology, it has been pointed out that bronchi may be identified within 1 cm of the mediastinal pleura in normal subjects, but that airways seen within 1 cm of the costal pleura or paravertebral pleura should be regarded as abnormal. 290 Large plugged bronchi are visible as lobulated or branching opacities – such airways are usually seen in the presence of nonfluid-filled but obviously bronchiectatic airways. A less frequent pattern is plugging and thickening of the smaller peripheral centrilobular airways, producing V- and Y-shaped opacities, the so-called ‘tree-in-bud’ appearance307.308. and 309. (Fig. 12.28); similarly, this pattern is almost invariably accompanied by abnormal, if not frankly bronchiectatic, larger airways.
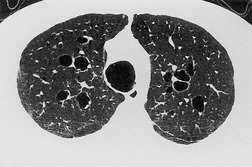
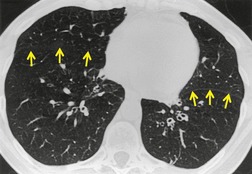
In many patients with bronchiectasis, areas of decreased attenuation of the lung parenchyma can be identified (Fig. 12.29); this mosaic attenuation pattern reflects accompanying obliteration of small airways disease.286. and 310. Sections taken at end-expiration enhance the feature of decreased attenuation, the extent of which correlates with functional indices of airways obstruction.310. and 311. This finding is most prevalent in lobes with severe bronchiectasis but may be seen in some lobes in which there are no CT features of bronchiectasis. Kang et al. 286 identified a mosaic pattern in just over half of bronchiectatic lobes subsequently resected, and there was pathologic evidence of obliterative bronchiolitis in 85% of these resected lobes. Areas of decreased attenuation on CT representing constrictive obliterative bronchiolitis may sometimes be confused with the features of emphysema. 312 Nevertheless, the rare association of bronchiectasis and true emphysema does sometimes occur. When a panacinar emphysema pattern (uniform decrease in attenuation of the lung parenchyma – see p. 763) is identified in the lower lobes, as part of α1-antitrypsin deficiency, it is often accompanied by bronchiectasis.313. and 314. Serial fluctuation in pulmonary function measures of airflow obstruction most closely correlate with alterations in the degree of mucus plugging on serial HRCTs. 315
Subtle degrees of volume loss may be seen in lobes in relatively early bronchiectasis and this is most evident in the lower lobes where crowding of the mildly bronchiectatic airways and posterior displacement of the oblique fissure may be an early sign of bronchiectasis (Fig. 12.30). CT will readily show completely collapsed lobes containing bronchiectatic airways although the diagnosis of bronchiectasis in acutely collapsed or consolidated lobes is usually considered uncertain because of the reversibility of bronchial dilatation in these situations.204. and 205. An interesting and not readily explained accompaniment to bronchiectasis is thickening of the interlobular septa; this occurs in up to 60% of patients with idiopathic bronchiectasis and appears to be more prevalent in cases of more severe and extensive bronchiectasis316 (Fig. 12.31).
Distortion and dilatation of segmental and subsegmental bronchi are predictable features in patients with retractile interstitial fibrosis, 317 of whatever cause, and this has been termed (not particularly usefully) ‘traction bronchiectasis’. Many of the HRCT signs of bronchiectasis coexist, particularly in longstanding disease. The signs of bronchiectasis, discussed above, are summarized in Box 12.13 in the approximate sequence in which they occur.
Box 12.13
• Bronchial wall thickening
– Nonspecific and variable
• Lobar volume loss
– May be minimal; most obvious in the lower lobes
• Bronchial dilatation
– Signet ring sign or nontapering bronchiectasis
– The cardinal sign of bronchiectasis
• Mosaic attenuation pattern
– Reflecting coexistent constrictive bronchiolitis, may be an early feature
• Tree-in-bud pattern
– Representing exudate in and around small airways
• Mucus plugging of large airways
– A late sign
• Interlobular septal thickening
– An occasional feature linked to the severity of bronchiectasis
There are several situations in which the signs of bronchiectasis may be obscured by technical artifacts, or mimicked by other lung pathologies,287. and 288. and these are summarized in Chapter 4 (Box 4.9, p. 189).
Accuracy of HRCT for the detection of bronchiectasis
In the absence of a reliable gold standard, the accuracy of HRCT in confirming or excluding bronchiectasis is difficult to ascertain. Evidence from early CT studies (using widely different technical parameters, particularly section collimation278.318.319. and 320.) does not allow any definite conclusions to be drawn, particularly as bronchography (the then gold standard) cannot be regarded as a wholly reproducible technique.321. and 322. In one study in which thin-section CT findings were compared with pathologic features, from surgically resected bronchiectatic lobes, Kang et al. 286 showed that CT identified bronchiectasis in 41 (87%) of 47 lobes with pathologically proven bronchiectasis. Because the patients selected for this study had relatively severe disease, the authors suggested that the detection rate of CT may be less in patients with mild bronchiectasis. However, the less than perfect performance of HRCT in this study might have reflected the fact that the deranged state of scarred lungs in end-stage bronchiectasis precludes the reliable identification of bronchiectatic airways. In the lobes considered to have bronchiectasis on CT, the most frequently identified sign was a lack of tapering of the bronchial lumens (37/41), followed by bronchial wall thickening (32/41), bronchial dilatation (28/41), identification of bronchi in the lung periphery (21/41), and mucus-filled dilated bronchi (3/41). The causes of the 9/47 (13%) false negatives were due to either masslike lesions or areas of dense opacification or scarring in which dilated bronchi were not identifiable. In a further study that compared the CT features of patients with surgically proven or CT-diagnosed bronchiectasis with normal subjects, lack of tapering of the bronchi was identified in 95% of patients with bronchiectasis compared with 10% of healthy subjects. 290 The results of these studies reinforce the observation of Lynch et al. 294 that lack of bronchial tapering should be regarded as the most reliable CT feature of bronchiectasis.
Disease-specific patterns of bronchiectasis
In some patients, the cause for bronchiectasis may be deduced from ancillary CT features. For example, the diagnosis of Swyer–James (McLeod) syndrome (p. 750) can be readily made from the asymmetric involvement and additional features of decreased attenuation and reduced pulmonary vasculature of the ipsilateral lung.323. and 324. Similarly, the diagnosis of α1-antitrypsin deficiency may be suggested by the combination of widespread cylindrical or cystic bronchiectasis and panacinar emphysema in the lower lobes.313. and 314. However, an underlying cause for bronchiectasis is found in less than half of patients213. and 215. and CT features alone do not usually allow a confident distinction between idiopathic bronchiectasis and a known cause of bronchiectasis.295. and 325. Nevertheless, in some cases the pattern and lobar distribution of bronchiectasis may be sufficiently characteristic for a specific underlying cause to be diagnosed.326.327. and 328. For example, the bronchiectasis of allergic bronchopulmonary aspergillosis is typically upper zone and central in distribution, with more normal distal bronchi. These features may be helpful in distinguishing this condition from other causes or idiopathic bronchiectasis.329.330.331. and 332.
Several other distinctive, if not diagnostic, patterns have been described in patients with a known cause of bronchiectasis. A lower and middle lobe distribution of cylindrical bronchiectasis with particularly marked bronchial wall thickening is typical in patients with hypogammaglobulinemia,222. and 333. and in patients with common variable immune deficiency there is often a background reticular pattern reflecting granulomatous fibrosis. 243 The upper lobe predilection for the cylindrical bronchiectasis of cystic fibrosis is well known.235.334. and 335. In patients with bronchiectasis due to nontuberculous mycobacterial infection, there is usually an accompanying nodular pattern;336. and 337. indeed, there appear to be some differences in patterns of disease between the subspecies of nontuberculous mycobacteria. 338 Apart from the localized bronchiectasis associated with post-tuberculous fibrocalcific damage, the range of possible manifestations of post-tuberculous bronchiectasis remains poorly documented. Idiopathic bronchiectasis has been reported to be predominantly basal in distribution.295. and 327. However, studies that have sought to determine whether observers can reliably distinguish between idiopathic bronchiectasis and bronchiectasis of known cause have not been conclusive and suggest that, although several CT features occur more frequently in certain groups of patients with an identifiable underlying cause, none of the CT features evaluated can be regarded as pathognomonic.295.325. and 328.
Some conditions with more or less distinctive features on HRCT (including the distribution of disease, pattern of bronchiectasis, and ancillary features) are listed in Box 12.14.
Box 12.14
• Allergic bronchopulmonary aspergillosis
• Swyer–James (McLeod) syndrome
• Tracheobronchomegaly (Mounier–Kuhn syndrome)
• α1-Antitrypsin deficiency
• Cystic fibrosis
• Mycobacterium avium–intracellulare complex
Cystic fibrosis
Cystic fibrosis, also known as cystic fibrosis of the pancreas, fibrocystic disease, and mucoviscidosis, is the most common autosomal recessive disorder in the Caucasian population, with a frequency of about 1 in 2500 livebirths.339. and 340. The disease is uncommon in African Americans and rare in Asians and Native Americans. Worldwide, 60 000 people are affected. 341 Cystic fibrosis is caused by a mutation in a gene on chromosome 7 that codes for the cystic fibrosis transmembrane conductance regulator (CTFR).342. and 343. Although over 800 mutations have been identified, 344 the most common mutation that causes cystic fibrosis is known as delta-F508. 342 CFTR functions primarily as a chloride ion channel and the defective protein affects pancreatic function and the consistency of mucosal secretions. Although the defective gene has been identified, gene replacement therapy is still far from clinical realization. 345
The primary manifestations of cystic fibrosis include abnormal sweat electrolytes, sinus and pulmonary disease, exocrine pancreatic insufficiency, and male infertility. 346 However, most organ systems are affected, to a greater or lesser extent, because of the wide tissue distribution of CFTR, but the lungs bear the brunt of the disease. Despite major advances in treatment, pulmonary infection remains the major cause of morbidity and mortality.
Staphylococcus aureus and Haemophilus influenzae are the most common infecting organisms in the first decade of life, but infections caused by Pseudomonas and Burkholderia species dominate thereafter. By adulthood, 80% of patients are colonized with P. aeruginosa and 3.5% with Burkholderia cepacia, Stenotrophomonas maltophilia, and Achromobacter xylosoxidans. Nontuberculous mycobacteria are emerging as important pathogens in patients with cystic fibrosis, particularly Mycobacterium abscessus, M. avium–intracellulare, Mycobacterium fortuitum and Mycobacterium kansasii.347. and 348. There are difficulties in interpreting the importance (colonization versus infection) of the presence of these organisms when they are isolated from the sputum of patients with cystic fibrosis, 349 especially because of the similarity in HRCT appearances of cystic fibrosis with or without nontuberculous infection.
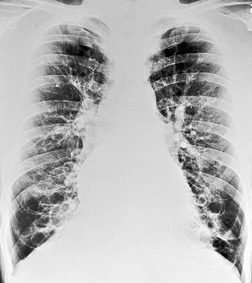
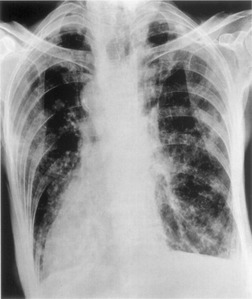
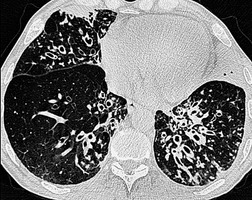
Although the disease is present at birth, radiographic abnormalities may not become apparent for months or years, but thereafter are progressive (Fig. 12.32). The earliest findings are nonspecific and include diffuse bronchial wall thickening, focal atelectasis, and recurrent pneumonia. At this stage, the clinical features of the disease, not the chest radiographic findings, suggest the diagnosis. Progression of disease with worsening radiographic abnormalities is usually inexorable, despite treatment, but the rate of deterioration is highly variable. Patients with early manifestations of the disease tend to deteriorate the most rapidly. 350 In the fully developed form of the disease the radiographic findings are remarkably uniform and include the following:351.352.353.354. and 355.
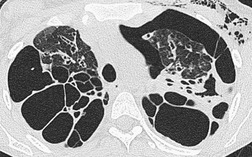
• Bronchial wall thickening and bronchiectasis – which result from chronic inspissation of mucus and airway infection, are the hallmarks of the disease (Fig. 12.32D). These findings are seen on chest radiographs as peribronchial cuffing, tram-tracking and ring shadows. Associated mucoid impactions that manifest as ‘finger-in-glove’, ‘toothpaste’, or nodular opacities are also common. Bronchiectasis may be cylindrical or cystic depending upon the severity and chronicity of the disease. Cystic bronchiectasis may be an impressive feature of the disease (Fig. 12.33). The cysts can be up to 3 cm in diameter and usually have thin walls. The walls may be thickened by associated infection and the cysts may contain variable quantities of fluid. In advanced disease it may be impossible to distinguish between cystic lung destruction and grossly dilated airways, particularly in the upper lobes.
• Atelectasis and focal consolidation – these are also common and may wax and wane in association with acute infection. Extensive cicatricial atelectasis in the upper lobes may result from chronic or recurrent infection (Fig. 12.34). Large areas of consolidation are uncommon but are seen in overwhelming, usually P. aeruginosa, infection or, more frequently, allergic bronchopulmonary aspergillosis283. and 356. (Fig. 12.35).
• Enlarged hila and diffuse perihilar opacities – enlargement of the hila is caused by lymphadenopathy, dilatation of the pulmonary arteries (reflecting pulmonary hypertension), and inflammatory changes adjacent to the hila (Fig. 12.36). Lymphadenopathy, presumably due to reactive hyperplasia, is a common finding in patients with cystic fibrosis. 355 Diffuse perihilar opacities are due to peribronchial inflammation and central bronchiectasis (Fig. 12.36), and in some individuals may reflect coexisting allergic bronchopulmonary aspergillosis.
• Progressive airway obstruction results in pulmonary hyperinflation – the thorax becomes barrel-shaped with an increased anteroposterior diameter (Fig. 12.32E). The diaphragms are usually low and flattened.
Parenchymal findings seen on chest radiographs of patients with longstanding cystic fibrosis are usually more severe in the upper than lower lungs (Fig. 12.34). Nevertheless, in younger children this upper lobe predominance may be less noticeable. 357
Chest radiographs over a number of years generally reflect the patient’s deteriorating status, but short-term clinical fluctuations are not necessarily accompanied by any readily detectable radiographic changes. Greene et al. 358 studied 14 specific radiographic findings in a group of adults with and without acute exacerbations of cystic fibrosis. They were unable to find a statistically significant association between any of the findings and the presence or absence of acute symptoms. These authors concluded that the value of the chest radiograph in the setting of an acute cystic fibrosis exacerbation lay more in excluding a major complication such as pneumothorax. In part, this must reflect the inherent difficulties in detecting small focal radiographic abnormalities against a background of diffuse bronchiectasis and lung destruction. Nevertheless, a number of scoring systems use findings on chest radiographs to grade the severity of cystic fibrosis.351. and 359.
Common complications of cystic fibrosis include pneumothorax and hemoptysis. Pneumothorax is caused by rupture of emphysematous blebs or bullae. 360 Hemoptysis usually originates from hypertrophied bronchial arteries and varies in severity from minor blood-streaked sputum to massive hemoptysis (greater than 240 mL/24 h). 361 Affected patients tend to have very severe lung disease and a high incidence of infection by multidrug-resistant bacteria. 361 Bronchial artery embolization (BAE) is an effective treatment for cystic fibrosis patients with pulmonary bleeding.361.362. and 363. Brinson et al. 361 reviewed their experience with BAE in 18 patients treated over a 10-year period. They found that the overall efficacy of BAE for initial control of bleeding was 75% after one, 89% after two, and 93% after three treatments. Unfortunately, they also found that the rate of recurrent bleeding was high (46%); the mean time to recurrence was approximately 12 months. They also found a high incidence (75%) of bleeding from nonbronchial systemic collateral vessels in the patients who had undergone previous BAE, indicating the necessity of evaluating all potential systemic collateral vessels. Lung abscess and empyema are surprisingly uncommon complications of cystic fibrosis.
The CT findings of cystic fibrosis on conventional364 and thin-section CT have been extensively documented.235.236.334.365.366.367.368.369. and 370. As might be expected, CT delineates the morphologic changes of cystic fibrosis with much greater accuracy and detail than chest radiographs. As with chest radiography, CT has only limited usefulness in the investigation of patients with acute exacerbations of cystic fibrosis. The only finding in the study of Shah et al. 368 that had a correlation with acute exacerbation was the presence of air–fluid levels. Another study has confirmed that changes in mucus plugging on HRCT are the most labile abnormality in acute exacerbations. 371 Brody et al. 372 have shown that scores of severity of disease on CT over a period correlate with the number of exacerbations in that period, although no CT feature reliably predicted exacerbations.
Numerous investigators have reported moderate to good correlation between functional impairment and CT findings using a variety of CT scoring methods.236.357.365.369.373.374.375.376. and 377. While these methods for quantifying disease severity may be useful from a research standpoint, potentially providing surrogate endpoints in clinical trials, the usefulness of routine monitoring of individuals with cystic fibrosis with CT in everyday clinical practice remains controversial.378.379. and 380.
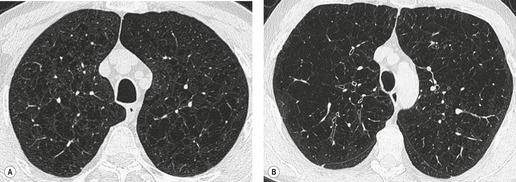
The CT findings of CF vary with duration and severity of disease.236.357.365.369.370.373.374.381. and 382. Bronchiectasis is the predominant abnormality. Cylindrical bronchiectasis is more common than cystic bronchiectasis, particularly in patients with mild lung disease.235. and 334. Over time the bronchiectasis progresses and HRCT may depict changes in the face of stable or minimal decline in lung function382. and 383. (Fig. 12.37). In advanced cases, both cylindrical and cystic forms of bronchiectasis tend to be more severe in the upper lungs (Fig. 12.38). Bronchial and peribronchial thickening is also a common finding on CT384 that reflects the chronic inflammatory changes in the bronchial wall. Mucus plugs, a very common finding, are seen to advantage with CT (Fig. 12.39). Abscesses may be difficult to distinguish from cystic bronchiectasis, particularly as both may contain air–fluid levels. Other CT findings include: focal areas of collapse or consolidation; tree-in-bud pattern; mosaic attenuation; bullae, hilar, and mediastinal adenopathy; and pleural thickening. Bullae may be difficult to distinguish from cystic bronchiectasis, particularly in fibrotic upper lobes (Fig. 12.40). A mosaic attenuation pattern likely reflects obstruction of small airways (Fig. 12.40). Pleural thickening is often apparent on chest radiographs and is demonstrated to advantage by CT. 235 The extent of such thickening may have some bearing on difficulties at lung transplantation, although it has been convincingly shown that assessment with pretransplantation CT is, in fact, of little value. 385 In some patients who develop a pneumothorax CT may usefully identify the optimal site for chest drain insertion because the visceral pleura is often tethered to the chest wall as a result of pleural adhesions386 (Fig. 12.41). The CT features of cystic fibrosis are summarized in Box 12.15.
Box 12.15
• Bronchiectasis
– Cylindrical or cystic, upper lobe predominant
• Peribronchial thickening
• Mucus plugging
– Tree-in-bud pattern through to bronchoceles
• Mosaic attenuation pattern
• Consolidation and atelectasis
– Consolidation may be noninfective (e.g. allergic bronchopulmonary aspergillosis [ABPA])
• Bullae
• Pleural thickening
– Usually limited pleural ‘tags’ in the upper zones posteriorly
MRI has been used for investigation of patients with cystic fibrosis;387. and 388. it can demonstrate many of the salient features of cystic fibrosis, but lacks the spatial resolution of CT. Donnelly et al. 344 used hyperpolarized 3He-enhanced MRI to image patients with cystic fibrosis. The technique is quite sensitive for small airway obstruction389 and can provide a means of evaluating progression of disease in patients with cystic fibrosis without use of ionizing radiation, a use which is likely to be limited to research applications.
Ciliary dyskinesia syndrome (immobile cilia syndrome) (Box 12.16)
The ciliary dyskinesia syndrome (CDS) is an example of one of many specific causes of bronchiectasis, and with changing patterns of etiology it is becoming relatively more important. In a selected series of patients referred to a specialist hospital for investigation of chronic cough and sputum production, ciliary dyskinesia was, after postinfection bronchiectasis, the most frequent etiology, 390 but was less prevalent in another series. 215 In, ciliary dyskinesia, first identified in 1976, 391 a variety of genetically determined defects in ciliary structure and function392 interfere with mucociliary clearance. 393 Impaired mucus clearance is associated with recurrent upper and lower respiratory tract infections. 394 CDS shares many features with cystic fibrosis but is less disabling and carries a better prognosis.282. and 395. Kartagener syndrome396– situs inversus, paranasal sinusitis, and bronchiectasis – is a subtype of CDS, and about 50% of patients with CDS have Kartagener syndrome282.394. and 397. (see Fig. 12.21). About one-fifth of subjects with dextrocardia have Kartagener syndrome. 398 CDS has autosomal recessive transmission with an equal sex incidence. In Europe and the USA CDS has a prevalence of about 1 : 20 000 individuals. 399 Ciliary function is abnormal throughout the body, and sperm are immotile; thus males are infertile. Fertility in females is generally unaffected, although there are exceptions. 397 Respiratory symptoms may be delayed in onset but can generally be traced back to childhood, and CDS is increasingly recognized as a cause of neonatal respiratory distress.400. and 401. Symptoms are those of bronchitis, rhinitis, and sinusitis, which are universal, and otitis, which is less common. Bronchiectasis develops in childhood and adolescence and is associated with recurrent infections. Prognosis is generally good, and the diagnosis is compatible with a full lifespan. 398 The diagnosis is regarded as established in the following circumstances: (1) complete Kartagener syndrome, (2) men with normal situs but a classic history and immotile sperm, (3) women and children with normal situs but typical history and an affected sibling, and (4) subjects with normal situs but with a classic history and ultrastructural defects of nasal or bronchial cilia on biopsy.394. and 402. Findings on the chest radiograph and CT are of bronchial wall thickening (almost invariably) and bronchiectasis with a predilection for involvement of the middle lobe and lingula403 (Fig. 12.42). In a few patients with CDS there is abnormal calcium deposition within the affected airways, such that this may be visible on imaging; lithoptysis (coughing up of broncholiths) is also a rare association. 404
Box 12.16
• One of the commonest identifiable causes of bronchiectasis in the pediatric age range
• Associated with infertility in males
• Presentation with bronchiectasis may be delayed until adulthood
• Accompanying sinusitis is invariable
• HRCT pattern of bronchiectasis is cylindrical with a predilection for the right middle lobe and lingula
Young syndrome266. and 267. clinically resembles CDS. However, in Young syndrome ciliary function is normal and infertility is due to obstructive azoospermia. Obstruction occurs at the level of the epididymis, which is palpably enlarged. The pathogenesis of increased sinopulmonary infection in these patients is obscure.
BRONCHOLITHIASIS (Box 12.17)
The term ‘broncholithiasis’ is generally interpreted more widely than meaning simply the condition resulting from endobronchial calcified material. 405 Most authors include, in addition, the effects of airway distortion or inflammation caused by calcified peribronchial nodes406 or other rarer causes of focal calcific endoluminal lesions. 407 Nearly all cases are due to infective nodes, particularly following histoplasmosis.408. and 409. Other causal infections include tuberculosis, actinomycosis, coccidioidomycosis, and cryptococcosis. A few cases have been reported with silicosis410 and in association with ciliary dyskinesia. 404 Calcified material in an airway or luminal distortion caused by peribronchial disease results in airway obstruction. This in turn leads to collapse, obstructive pneumonitis, mucoid impaction, or bronchiectasis. Rarely fistulas can form from the airway to the esophagus, 411 pleural space, or aorta408 and mediastinal abscess formation has been reported. 412 Symptoms commonly include cough, hemoptysis, and recurrent episodes of fever and purulent sputum.226. and 408. The classic symptom of lithoptysis is uncommon, with a reported frequency of between 13% and 16%.226. and 408.
Box 12.17
• There are many causes of a calcified endobronchial obstructing lesion, the commonest result from granulomatous infections
• Consequences include postobstructive atelectasis, mucoid impaction, and bronchiectasis
• Diagnosis on chest radiography may be difficult in the absence of heavy calcification of the broncholith
• CT with reconstructions and/or bronchoscopy are needed to make the diagnosis of broncholithiasis
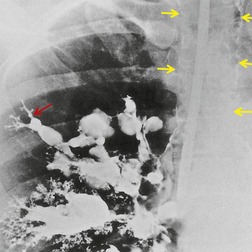
In a review of the plain radiographic findings three major types of change were distinguished: 405 (1) disappearance of a previously identified calcified nidus; (2) change in position of a calcified nidus; and (3) evidence of airway obstruction including segmental or lobar atelectasis, mucoid impaction, obstructive pneumonitis, and obstructive overinflation with air trapping. Predictably there may be signs of bronchiectasis. Calcified hilar or mediastinal nodes are a key feature of the radiograph, and it is important to inspect all calcifications, assess their position, and look for evidence of movement on serial radiographs. Movement can be difficult to detect and may just be a relatively subtle rotation. 405 Broncholithiasis is more common on the right, 408 and obstructive changes particularly affect the right middle lobe.
The diagnosis may not be suspected on chest radiography. CT and fiberoptic bronchoscopy complement each other in this condition, neither on its own being necessarily diagnostic. Principal findings on CT226.407.413.414. and 415. are a calcified lymph node within an airway or immediately adjacent to a distorted airway, distal changes secondary to bronchial obstruction, and absence of an associated soft tissue mass (Fig. 12.43, see also Fig. 5.63, p 236). CT is not always correct in accurately localizing calcification because of partial volume effects, and in one series 40% of truly endobronchial lesions were interpreted on CT as extrabronchial. 226 Reconstruction of volumetric CT data, including three-dimensional depictions, 416 can be used to overcome this problem. 407 The CT appearance of broncholithiasis can be mimicked by calcified endobronchial hamartomas or carcinoid tumors. 417
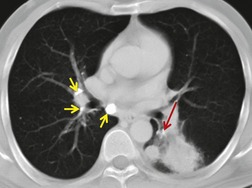 |
| Fig. 12.43 (With permission from Seo JB, Song KS, Lee JS, et al. Broncholithiasis: review of the causes with radiologic-pathologic correlation. RadioGraphics 2002;22:S199–213. Copyright Radiological Society of North America.) |
SMALL AIRWAYS DISEASES
Bronchiolitis includes a spectrum of inflammatory and fibrosing disorders that predominantly affect the small airways (terminal and respiratory bronchioles). Diseases affecting the small airways show great variability as regards cause, clinical features, and histopathologic changes. A number of attempts to classify these conditions have been made,418.419.420.421.422. and 423. and one of the more comprehensive schemes, described by Myers and Colby, 420 is shown below:
1. Constrictive bronchiolitis (obliterative bronchiolitis, bronchiolitis obliterans)
2. Cryptogenic organizing pneumonia (bronchiolitis obliterans organizing pneumonia [BOOP], proliferative bronchiolitis)
3. Acute bronchiolitis (infectious bronchiolitis)
4. Small airways disease (adult bronchiolitis)
5. Respiratory bronchiolitis (smoker’s bronchiolitis, respiratory bronchiolitis-associated interstitial lung disease)
6. Mineral dust airways disease (early pneumoconiosis)
7. Follicular bronchiolitis
8. Diffuse panbronchiolitis.
Diseases affecting the small airways are difficult to detect by conventional radiographic and physiologic tests; widespread involvement occurs before symptoms or abnormalities on pulmonary function testing become apparent. An understanding of the distribution of disease in relation to the airways at a pathologic level allows some prediction of the likely CT appearances in this wide spectrum of conditions, and thus helps to refine differential diagnosis.
The difficulty in detecting small airways dysfunction on pulmonary function testing can be readily appreciated by considering the fact that the summed cross-sectional area of the small airways luminal diameters is much greater than that of the central airways and so accounts for less than a quarter of total airflow resistance. Thus an extraordinary number of small airways need to be affected before there is a measurable physiologic effect on airflow limitation.
The terminology used to classify diseases of the small airways is confusing but can, for imaging purposes, be greatly simplified. Manifestation of small airways disease on HRCT can be broadly categorized into direct and indirect signs: considerable thickening of the bronchiolar walls by inflammatory infiltrate and/or luminal and surrounding exudate render affected airways directly visible (tree-in-bud pattern). By contrast, cicatricial scarring of many bronchioles results in the indirect sign of patchy density differences of the lung parenchyma, the areas of decreased attenuation reflecting areas of underventilated, and consequently underperfused, lung (mosaic attenuation pattern); these two basic patterns of small airways disease are more fully discussed in Chapter 4.
Pathologic classification and clinical background
Inflammation of the bronchioles (bronchiolitis) with or without subsequent scarring and obliteration is a very common lesion in the lungs. 424 However, the extent of such lesions is rarely extensive enough to cause clinical symptoms. Texts in the pathology often emphasize the frequent involvement of the bronchioles in diverse diffuse lung diseases.
The specific and classical term obliterative bronchiolitis (synonymous with bronchiolitis obliterans) has historically been the subject of confusion, primarily because of its use in the context of BOOP. The clinicopathologic entity of BOOP, now termed cryptogenic organizing pneumonitis,425. and 426. is now regarded as quite distinct from obliterative bronchiolitis (Fig. 12.44). There are several reports in the older literature describing cases of ‘bronchiolitis obliterans’ whose pathologic descriptions contain all the hallmarks of an organizing pneumonia (characterized by buds of loose granulation tissue occupying the airspaces and respiratory bronchioles, without any obliteration of the small airways).427.428. and 429. It is now generally accepted that there is no direct connection between obliterative bronchiolitis and BOOP, 430 such that in the interests of clarity most authorities suggest that the bronchiolitis obliterans part of BOOP should be discarded. In those patients in whom no causative agent for the organizing pneumonia can be found, the term cryptogenic organizing pneumonia is more appropriate426 (see p. 575).
The various conditions included within the term ‘small airways diseases’ are usually classified into pathologic subtypes or by less precise clinical criteria (usually by presumed cause or association). While pathologists categorize small airways diseases according to their histopathologic subtypes, the difficulty with this classical approach is that there are not always obvious clinical (or HRCT) correlates with these subtypes.431.432.433. and 434.
A simple approach relies on the fundamental difference between the indirect HRCT signs of constrictive obliterative bronchiolitis and the direct visualization of exudative forms of bronchiolitis (typified by diffuse panbronchiolitis). 435 These two patterns of small airways disease account for the majority encountered in clinical practice. Other miscellaneous forms of small airways disease with more or less distinctive pathologic and imaging features, if not clinical presentations, are dealt with separately.
Constrictive obliterative bronchiolitis
Constrictive obliterative bronchiolitis (hereafter referred to as constrictive bronchiolitis) is, as its name implies, a condition characterized by bronchiolar and peribronchiolar inflammation and fibrosis that ultimately leads to luminal obliteration.418.420. and 436. The early change is a cellular inflammation that is intraluminal, mural, and peribronchial, affecting membranous and respiratory bronchioles. Inflammatory cells are a mixture of neutrophils, lymphocytes, and plasma cells. The mature lesion is a peribronchiolar fibrosis, encroaching on the lumen with eventual occlusion of the airway. 420 Other features include smooth muscle hyperplasia and bronchiolectasis with mucoid impaction. 418
Given the close proximity of pulmonary arterioles, some inflammatory involvement is to be expected; however in later stages remodeling of these vessels, probably in response to chronic underventilation and hypoxia, becomes evident (an irreversible phenomenon, not to be confused with ‘reversible’ hypoxic vasoconstriction encountered in, for example, the bronchospasm of acute asthma).
Clinical findings are extremely variable in severity and vary according to cause and severity, but symptoms typically consist of progressive dyspnea and nonproductive cough unaccompanied by significant wheezing. On auscultation of the chest, crackles and, apparently characteristic, inspiratory squeaks and squawks are heard. Pulmonary function tests show airflow obstruction, sometimes with restriction, with a normal gas transfer adjusted for alveolar volume (Kco).437. and 438. There is evidence of gas trapping with a low forced vital capacity and a high residual volume. Airflow limitation is volume-dependent and may be demonstrated by use of flow volume loops that allow calculation of maximum midexpiratory flow rates (MMEFRs). However, a reduced MMEFR is not specific for small airways disease. 439
Constrictive bronchiolitis is a relatively uncommon disorder that in most cases is associated with a recognized cause and is rarely truly cryptogenic. Reported causes and associations are listed in Box 12.18.
Box 12.18
• Postinfection
• Mycoplasma445
• Nitrogen dioxide (silo-filler disease), sulfur dioxide, ammonia, chlorine, phosgene448
• Mustard gas449
• Hot gases450
• Connective tissue disorders439
• Others very rarely
• Allograft recipients
• Drugs
• Penicillamine461
• Cryptogenic437 (truly idiopathic cases very rare)
• Other conditions
• Chronic bronchitis464
• Cystic fibrosis465
• Hypersensitivity pneumonitis466
• Sauropus androgynus ingestion469
• Popcorn production workers470
From the radiologist’s viewpoint it may be useful to consider the general situations in which the HRCT signs of constrictive bronchiolitis may be encountered, and their significance (see Box 12.19).
Box 12.19
• Trivial extent – e.g. subclinical post mild viral lower respiratory tract infection; asthma; healthy individuals
• Expected – e.g. bronchiectasis; cigarette smoker
• Component of chronic interstitial lung disease – e.g. sarcoidosis; hypersensitivity pneumonitis
• Dominates clinical picture – e.g. post-transplantation; following severe viral pneumonia; rheumatoid arthritis; fume inhalation
Viral infections, particularly by respiratory syncytial virus and adenovirus,441. and 471. are a common cause of constrictive bronchiolitis in children. Nonviral agents are much less commonly implicated, although Mycoplasma pneumoniae is a particularly potent cause of constrictive bronchiolitis440.444. and 445. (Fig. 12.45). Caution is needed in interpreting reports that suggest bacterial infections, for example Nocardia asteroides and Legionella pneumophila, may be responsible for constrictive bronchiolitis:429. and 472. the pathology described in these particular reports is of an organizing pneumonia rather than constrictive bronchiolitis (highlighting the historical confusion surrounding the terminology of ‘bronchiolitis obliterans’). Postinfectious constrictive bronchiolitis is largely confined to children; although repeated viral lower respiratory tract infections are a usual fact of adult life, clinically important constrictive bronchiolitis as a consequence is fortunately rare473 (Fig. 12.46). Swyer–James (or McLeod) syndrome is a particular form of constrictive bronchiolitis that occurs following an insult, usually a viral infection, to the developing lung and this is discussed on pages 750–752.
 |
| Fig. 12.46 (Modified with permission from Green M, Turton CW. Bronchiolitis and its manifestations. Eur J Respir Dis Suppl 1982;121:36–42.) |
Constrictive bronchiolitis is a predictable consequence of the inhalation of many toxic fumes and gases which reach the small airways. 447 It has been most frequently described following nitrogen dioxide inhalation (silo-filler disease),474. and 475. and more recently in individuals exposed to mustard gas in war zones. 449
Among the connective tissue diseases, constrictive bronchiolitis is most strongly associated with rheumatoid arthritis.439.451.476. and 477. Constrictive bronchiolitis associated with rheumatoid arthritis is occasionally rapidly progressive with refractory airflow obstruction unresponsive to any treatment. 451 Nevertheless, minor degrees of constrictive bronchiolitis are probably present and subclinical in many patients with rheumatoid arthritis.272.478. and 479. In historical reports about half of patients with rheumatoid arthritis and constrictive bronchiolitis had been taking penicillamine,437. and 451. and a cause and effect relationship was proposed.480. and 481. This was supported by a study of 602 patients with rheumatoid arthritis in which there was a 3% prevalence of constrictive bronchiolitis in patients receiving penicillamine but there were no cases of constrictive obliterative bronchiolitis in those not taking the drug. 461 Furthermore, a close temporal relationship often exists between starting penicillamine medication and the onset of symptoms. 439 It remains possible that penicillamine treatment is merely a ‘marker’ of those patients with more severe rheumatoid arthritis, who are thus more likely to develop complications of the disease, including constrictive bronchiolitis. Studies using indirect measures, such as pulmonary function testing and HRCT, suggest that small airways disease is probably more prevalent than earlier authors indicated.477. and 478. Patients with Sjögren syndrome may have a combination of interstitial disease (usually lymphoid interstitial pneumonia) and airways disease but, unlike rheumatoid arthritis, constrictive bronchiolitis is rarely the dominant presenting feature.453. and 482.
Constrictive bronchiolitis is an important and frequent cause of morbidity and mortality in patients receiving heart and lung transplants.456.460.483.484. and 485. It has a prevalence of between 25% and 50% and usually manifests itself between 9 and 15 months (range 60 days to 5.6 years) after transplantation. 485 It is probable that subclinical damage to small airways epithelium occurs earlier, within the first few weeks following transplantation. Subtle abnormalities on HRCT may predate the functional abnormalities of supervening small airways obliteration. 486 Nevertheless, the sensitivity of HRCT for the early detection of constrictive bronchiolitis in patients receiving transplants remains controversial,487.488.489. and 490. and the correlation between the functional severity of the so-called bronchiolitis obliterans syndrome and extent of CT abnormalities is not strong491 and some authors assert that the simple measure of monitoring FEV1 may be sufficient for detecting the development of clinically important constrictive bronchiolitis.492. and 493. Frequent and severe episodes of acute rejection, potentiated by cytomegalovirus and other infective agents, increase the risk of development of constrictive bronchiolitis. Modification of the immunosuppressive regimen may be successful in delaying the development of constrictive bronchiolitis, but relapses are common. 485 Constrictive bronchiolitis is also a well-recognized complication of bone marrow transplantation.456. and 494. The disorder develops usually within 18 months of transplantation and is also variably responsive to increased immunosuppression. 495
Constrictive bronchiolitis is rarely truly cryptogenic.437.496. and 497. Most reported cases probably have an undisclosed precipitating cause or association, such as a connective tissue disease which subsequently declares itself. 498
The radiographic features of constrictive bronchiolitis can be summarized as overinflation of the lungs, reduced pulmonary vasculature, with or without bronchial wall thickening499 (Fig. 12.47); these nonspecific abnormalities, seen in any form of chronic obstructive pulmonary disease, are prone to considerable observer variation. Furthermore, these signs are not present in all patients finally diagnosed as having constrictive bronchiolitis.294. and 500.
In an early CT study of constrictive bronchiolitis, 15 patients who fulfilled the criteria of Turton et al. 437 were examined with 10 mm section thickness and thin section CT (interspaced 3 mm sections). 496 Chest radiographs were normal in 5/15 patients; the remaining 10 patients showed ‘limited vascular attenuation and hyperinflation’. In 13/15 a pattern of ‘patchy irregular areas of high and low attenuation in variable proportions, accentuated in expiration’ was recorded; this, and a report of two cases by Eber et al., 501 were the first studies to identify inhomogeneity of the density of the lung parenchyma as the indirect, but key, CT feature of constrictive bronchiolitis.
Subsequent descriptions have confirmed and refined the HRCT features of constrictive bronchiolitis.438.502.503.504.505.506. and 507. The HRCT signs comprise patchy areas of reduced parenchymal attenuation (the ‘mosaic attenuation pattern’), reduction in the caliber of the pulmonary vessels within areas of decreased lung density, bronchial abnormalities (Fig. 12.48), and a relative lack of change of cross-sectional area of affected parts of the lung on sections obtained at end-expiration323. and 508. (Fig. 12.49). The individual HRCT signs of constrictive bronchiolitis, listed in Box 12.20, are considered in more detail in Chapter 4.
Box 12.20
• Areas of decreased attenuation (black lung)
• Reduction in caliber of the macroscopic pulmonary vessels within black lung
• Patchy involvement (mosaic attenuation pattern) unless advanced and end stage
• Abnormalities of the large airways – may be bronchiectasis or wall thickening
• Air-trapping seen as enhancement of the mosaic pattern at expiratory CT
The sensitivity and specificity of the sign of decreased attenuation for the diagnosis of constrictive bronchiolitis depend largely upon the context in which it is encountered: for example, in patients who have received lung transplants, one study has reported a sensitivity of 40%, and specificity of 78%, for the mosaic pattern. This increased to 80% and 94%, respectively, for the expiratory CT sections. 509 If the other HRCT features associated with constrictive bronchiolitis are also included (bronchial dilatation, bronchial wall thickening) 510 specificity, and to a lesser extent the sensitivity, of HRCT is increased. 509 Abnormalities of the macroscopic bronchi are a variable feature on HRCT, but are not unexpected given their anatomic continuity with the small airways; it seems that bronchial dilatation and bronchial wall thickening are relatively late features of constrictive bronchiolitis, and are more frequent in immunologically driven disease, such as rheumatoid arthritis511 (Fig. 12.50) or post lung transplantation. 512 The regional inhomogeneity of the lung attenuation may be extremely subtle in constrictive bronchiolitis, and is sometimes invisible on inspiratory CTs; the attenuation differences may be enhanced on CTs obtained at end-expiration484.508.513.514. and 515. (Fig. 12.51) or by postprocessing of the image data.516.517. and 518.
In some patients with extensive or end-stage constrictive bronchiolitis, the mosaic attenuation pattern may be absent. Furthermore, CT sections taken at end-expiration will appear remarkably similar to inspiratory sections: there will be no obvious change in cross-sectional area of the lungs (which normally decreases, at the level of the carina, by approximately 55%) 519 (Fig. 12.52). However, the generalized paucity of vessels and bronchial abnormalities will be present, although these findings may be very similar to those seen in widespread and severe panacinar emphysema.313. and 520.
The HRCT signs listed above are not, by themselves, specific for constrictive bronchiolitis and may be encountered in other chronic obstructive pulmonary diseases; for example, the mosaic attenuation pattern may be found in asthmatic patients, albeit usually less extensive than in patients with constrictive bronchiolitis. 521 However, in the context of a known cause or association of constrictive bronchiolitis, an HRCT showing this constellation of features can be regarded as diagnostic. Nevertheless, there are circumstances in which the distinction between constrictive bronchiolitis and other forms of obstructive pulmonary disease, particularly in patients with severe disease, can be difficult. 522 Areas of decreased attenuation of the lung parenchyma on HRCT in patients with constrictive bronchiolitis may sometimes be interpreted as ‘emphysema’; in constrictive bronchiolitis, the pulmonary vessels in affected lung are attenuated, but not distorted, as is the case in centrilobular emphysema. Furthermore, the extent of decreased attenuation caused by constrictive bronchiolitis does not correlate with gas diffusing capacity, the functional hallmark of emphysema. 438 However, the differentiation between panacinar emphysema (typified by patients with α1-antitrypsin deficiency) and advanced obliterative bronchiolitis may be less straightforward on HRCT appearances alone. Both conditions tend to show large areas of uniform, relatively featureless, decreased attenuation (‘black’) lung, and both are characterized by bronchial wall thickening and dilatation (Fig. 12.53). A quite useful discriminator is the presence of a ‘spider’s web’ of preserved interlobular septa just above the diaphragmatic surface in patients with panacinar emphysema (see Fig. 4.14, p. 175). A recent study that tested the ability of observers to distinguish, on the basis of HRCT findings, between cases of constrictive bronchiolitis, asthma, centrilobular emphysema, panacinar emphysema and normal individuals showed that the first choice diagnosis was correct in 199/276 (72%) observations, and agreement on distinguishing between cases of constrictive bronchiolitis and panacinar emphysema was reasonable (kappa 0.63). 522
Diffuse panbronchiolitis
Diffuse panbronchiolitis is the exudative small airways disease par excellence and is characterized by a tree-in-bud pattern at HRCT. 523 Diffuse (Japanese) panbronchiolitis is a sino-bronchial disease and was initially thought to be confined to Asian countries but sporadic cases have been reported in every continent; 524 nevertheless there appear to be predisposing racial and genetic (human leukocyte antigen [HLA]-associated genes) factors. 525 Symptoms include cough, sputum, chronic sinusitis and signs of progressive obstructive airways disease; given these invariable clinical features, it has been suggested that the inclusive term ‘sino-bronchial syndrome’ would be more appropriate, 526 but the original pathologic term diffuse panbronchiolitis is relatively unambiguous and well established. Some patients respond to long-term treatment with a macrolide, 527 although the exact mechanism of action of erythromycin is unknown (and is probably ascribable to its anti-inflammatory properties). 528 The prognosis may be poor with a reported 10-year survival rate as low as 25%. 529
Most of the definitive pathologic and imaging studies originate from Japan.307.530. and 531. The typical histopathologic features of diffuse panbronchiolitis are chronic inflammatory cell infiltration resulting in bronchiolectasis and striking hyperplasia of lymphoid follicles in the walls of the respiratory bronchioles; profuse foamy macrophages fill the bronchiolar lumens and the immediately adjacent alveoli, although the distal airspaces tend to be spared. This bronchiolocentric exudate is visible macroscopically as yellow nodules. As the disease progresses, an element of fibrotic bronchiolar constriction supervenes but, in the absence of longitudinal histopathologic studies, it is not clear the extent to which the basic ‘exudative’ pathology progresses to constrictive bronchiolar obliteration.
On chest radiography the dominant pattern is of numerous small (<5 mm) ill-defined nodules, such that the radiographic pattern may be interpreted as reflecting an interstitial, rather than an airway-centred, disease (Fig. 12.54). The nodules are symmetrically distributed and initially most prominent basally. Later, the radiographic features of cylindrical bronchiectasis may become evident. HRCT appearances reflect the pathologic distribution of disease: there is a nodular pattern and small branching opacities (tree-in-bud pattern) 532 can be identified in a predominantly centrilobular distribution, corresponding to the plugged and thickened small airways (Fig. 12.55). Accompanying cylindrical bronchiectasis, usually mild, is an almost invariable feature. Interestingly, although a mosaic attenuation pattern may be present in some cases, it is not usually a major feature; furthermore, air-trapping on expiratory CT is often surprisingly unimpressive. Nevertheless, functional studies have shown that the peripheral zone of lung is less dense than normal, presumably reflecting underventilation. 533 The individual HRCT signs of panbronchiolitis are summarized in Box 12.21.
Box 12.21
• Profuse tree-in-bud pattern (most obvious peripherally, more lower zone initially)
• Cylindrical bronchiectasis (mild)
• Mosaic attenuation pattern (inconspicuous or absent)
The HRCT features of diffuse panbronchiolitis, in the appropriate clinical setting, are virtually pathognomonic. However, other conditions may cause a similar (primarily tree-in-bud) pattern on HRCT532 and these include infections such as tuberculosis534. and 535. and invasive aspergillosis; 536 other causes of a tree-in-bud pattern are listed on page 192.
Miscellaneous conditions with small airways involvement
Hypersensitivity pneumonitis
Inhalation of organic dusts and deposition in the terminal and respiratory bronchioles causes an inflammatory (or cellular) bronchiolitis of variable severity in susceptible individuals. The potential for varying degrees of involvement of the airways and interstitium, and the coexistence of subacute and more chronic changes, explains the sometimes complex abnormalities found on pulmonary function testing. 537 The HRCT features of hypersensitivity pneumonitis are described in detail elsewhere (p. 457). With regard to the small airways involvement, there is a strong correlation between the extent of the areas of decreased attenuation (a component of the mosaic attenuation pattern) on HRCT and pulmonary function indices of air-trapping.466. and 538. The air-trapping, graphically shown on expiratory CT, is present in the majority of patients with subacute disease, and reflects the underlying component of cellular bronchiolitis (Fig. 12.56). Even in patients with chronic fibrotic disease, expiratory CT may show lobular air-trapping among the reticular pattern, likely reflecting fixed constrictive bronchiolitis (Fig. 12.57).
Sarcoidosis
By virtue of their perilymphatic distribution, sarcoid granulomas are concentrated around the airways. Physiologic studies have suggested that airflow obstruction located at the level of the small airways can occur in the early stages of sarcoidosis.539. and 540. Supportive evidence, seen as patchy air-trapping on expiratory CT was first described in three case reports, 541 and later confirmed in larger series542. and 543. (Fig. 12.58). In some cases the air-trapping, thought to reflect bronchiolar obstruction, foreshadows the more typical parenchymal manifestations of sarcoidosis. It seems that this phenomenon is common in patients with sarcoidosis at presentation (demonstrated in 20/21 patients in one series). 542 However, the prevalence of this phenomenon and its clinical significance has not been established.
Follicular bronchiolitis
Follicular bronchiolitis is primarily a histopathologic diagnosis and is characterized by hyperplastic lymphoid follicles, ranged along bronchioles that are compressed as a consequence. There is also infiltration of the adjacent bronchiolar walls and interstitium by polyclonal lymphocytes.544. and 545. The exact relationship between follicular bronchiolitis, lymphoid interstitial pneumonia and constrictive obliterative bronchiolitis, particularly in patients with rheumatoid arthritis in whom these pathologies may coexist, remains controversial.452. and 546. It is perhaps useful to consider follicular bronchiolitis as lying within the ambit of the low-grade lymphoproliferative disorders (most frequently encountered in the content of the connective tissue diseases). 547 Follicular bronchiolitis is most commonly encountered in patients with rheumatoid arthritis or Sjögren syndrome, but other associations include a familial form with immunodeficiency544 and HIV. 548 In one series of individuals with biopsy-proven follicular bronchiolitis, repeated lower respiratory infections were noted in 5/6549 and no predisposing cause (infections aside) for follicular bronchiolitis was identified in 4/6. The prognostic implication of follicular bronchiolitis (a diagnosis made on the basis of lung biopsy) is uncertain, particularly as it may be identified on a background of other pathology, for example usual interstitial pneumonia in association with a connective tissue disease. In some individuals, compression of the bronchioles by the hyperplastic follicles results in severe airflow limitation. 550 Peribronchial lymphoid hyperplasia in children (termed follicular bronchitis) may represent an exaggerated immune response to a viral infection, and results in mild airflow obstruction in the long term. 551 Follicular bronchiolitis appears to be a steroid-responsive disease. 549
The plain chest radiograph shows nonspecific small nodular or reticulonodular opacities,545. and 552. but may be normal. In an HRCT study of 14 patients (12 with a connective tissue disease) with biopsy-proven follicular bronchiolitis, the predominant abnormality was small nodules (3 mm diameter, but up to 12 mm in some cases). 553 In some cases the nodules had an obvious centrilobular and bronchocentric distribution, such that the HRCT pattern resembled sarcoidosis (Fig. 12.59). Areas of ground-glass opacification likely reflect the more generalized lymphocytic infiltration, present in just over half of patients. 553 Mild bronchial dilatation with wall thickening occurs in some cases (Fig. 12.59), but whether this is directly related to the presence of follicular bronchiolitis, or is associated with the background autoimmune disease, is unclear.
Respiratory bronchiolitis
The characteristic pathologic features of respiratory bronchiolitis in cigarette smokers were established many years ago and consist of an abundance of pigmented alveolar macrophages within the lumina of the respiratory bronchioles, and associated mild peribronchiolar interstitial fibrosis. 554 The term respiratory bronchiolitis-associated interstitial lung disease (RB-ILD) has been coined to describe a disease that is almost unique to cigarette smokers.555. and 556. Fuller consideration is given to this entity on page 451. In the very few individuals who develop a full clinicopathologic syndrome with symptoms ascribable to RB-ILD, the dominant pathologic abnormality is profuse intraalveolar macrophages, rather than bronchiolitis. The CT appearances are relatively nonspecific557.558. and 559. but in cases in which a history of heavy cigarette smoking is known, the CT findings can be highly suggestive of the diagnosis. The typical constellation of HRCT features include: patchy ground-glass opacification (reflecting the macrophage accumulation in the airspaces),300. and 560. poorly defined centrilobular nodules, 558 a limited reticular pattern (Fig. 12.60) with some thickening of the interlobular septa (probably due to accompanying minor interstitial fibrosis) and slight thickening of the macroscopic airways (possibly changes of chronic bronchitis) (Fig. 12.61). Emphysema is generally a minor feature, often with no more than a trace of paraseptal emphysema at the lung apices. In some individuals there is a mosaic attenuation pattern that may reflect the bronchiolitic element of the disease, 561 and/or a patchy DIP-like component. On expiratory CT, evidence of air-trapping is usually undramatic (Fig. 12.62), and not as extensive as that seen in other mixed interstitial and small airways diseases, such as subacute hypersensitivity pneumonitis.
Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia
Hyperplasia of neuroendocrine cells in the lungs is a very rare, but increasingly recognized, cause of obstructive lung disease.467.562. and 563. The cumbersome full name has been given the acronym DIPNECH, and this covers a spectrum of clinical presentations, ranging from asymptomatic individuals (a chance finding on histologic examination of a lung biopsy) through to a severe constrictive bronchiolitis-like syndrome.467. and 564. In most cases there is no obvious cause, although DIPNECH seems to occur more frequently in individuals with recurrent lower respiratory tract infections. In florid cases, in addition to the peribronchiolar fibrosis that characterizes the severe end of the DIPNECH spectrum, focal nodular aggregates of the neuroendocrine cells, termed micro-carcinoid tumorlets, develop. In a few individuals DIPNECH may occur as a backdrop in the setting of a typical carcinoid tumor.565. and 566. Because of the rarity of the condition, generalizations about the clinical profile cannot be made, but individuals affected by DIPNECH tend to be middle-aged women, often diagnosed as having asthma, with respiratory symptoms of many years’ duration. 563 The functional consequences of the resulting constrictive obliterative bronchiolitis may be very severe. In symptomatic patients with DIPNECH, HRCT may occasionally be normal but in the majority the key finding is a mosaic attenuation pattern,468. and 563. enhanced on expiratory CT. There may be accompanying bronchial wall thickening and nodules of varying sizes reminiscent of metastatic disease (although the tumorlets are not neoplastic in behaviour); close examination of the distribution of the larger nodules may show that they arise at the carinas of adjoining airways (the typical location of ‘conventional’ carcinoid tumours). The two HRCT signs of mosaicism and nodules (Fig. 12.63) are individually entirely nonspecific but, taken together in the context of a patient with disabling airflow limitation, they are suggestive of this curious and rare condition.
Swyer–James or McLeod syndrome (Box 12.22)
The Swyer–James or McLeod syndrome is a particular form of obliterative bronchiolitis that has special features: it occurs following an insult to the developing lung. The lung served by damaged bronchi and bronchioles remains inflated by collateral air drift. As defined in the original descriptions, the disease on chest radiography is predominantly unilateral, giving rise to the key finding of unilateral transradiancy.
Box 12.22
• Swyer–James syndrome results from viral injury to the developing lung (before the age of 8 years)
• Unilateral transradiancy on plain chest radiography in Swyer–James syndrome reflects a combination of hypoplasia of the pulmonary vasculature and obliterative bronchiolitis
• The affected lung is small or normal in volume
The condition was first described in the early 1950s, and it has been given a variety of noneponymous terms, including unilateral or lobar emphysema. However, these terms may lead to confusion with, for example, congenital lobar emphysema, and the current practice of using eponymous titles seems likely to continue. In previous editions of this book preference was given to ‘Swyer–James syndrome’ (Swyer and James published their report 1 year before McLeod). However, Müller, 567 in an intriguing account of chicanery, has made a persuasive case for giving ‘McLeod syndrome’ precedence.
The condition is characterized by bronchitis, bronchiolitis, constrictive obliterative bronchiolitis, and probably emphysema.568. and 569. Typically the condition is unilateral and a whole lung is affected, but changes may be confined to a lobe or segment. 570 Because it is usually an incidental finding on a chest radiograph performed for other reasons, there is no particular age at presentation; in one series the mean age was 38 years, and most (7/9) were male. 571 Other recognized patterns are of segmental sparing with the rest of the lung involved570 and of bilateral lobar or segmental disease. The patchy nature of lung involvement in some patients is particularly well demonstrated on CT examination. 572 Bronchi and bronchioles from the fourth generation to terminal bronchioles have submucosal fibrosis, causing luminal irregularity and occlusion.568. and 570. The lung parenchyma is hypoplastic, including the pulmonary artery and its branches, which are reduced in both size and number. Lung distal to diseased airways is hyperinflated and supplied by collateral air drift. Sometimes panacinar emphysematous changes are present, 573 although the definition of emphysema in the context of developing lung is controversial.
The Swyer–James syndrome is caused by injury of the immature lung. Injury most commonly follows an acute viral infection occurring during the first 8 years of life, before the lung has completed its development. 574 Viruses implicated include adenovirus575 and measles virus. 576 Nonviral causes include infections such as Mycoplasma pneumonia577 and pertussis.576. and 578.
Patients are typically asymptomatic and commonly present as adults with an abnormal chest radiograph. Less commonly patients have exertional dyspnea, which may be progressive and, exceptionally, quite marked, 569 or repeated respiratory infections.568. and 576. When coincidental acute lung disorders occur in the presence of the Swyer–James syndrome, the chest radiograph may show a unilateral distribution of acute abnormality, as is recorded with pulmonary edema579 and pulmonary hemorrhage. 580 Pulmonary function tests show a reduced vital capacity, airflow obstruction, and a reduced gas diffusing capacity.
Findings on the plain chest radiograph are characteristic. Unilateral transradiancy is caused by reduced lung perfusion. Lesser degrees of involvement are not easily detectable on the plain radiograph. On the affected side the size and number of mid-lung and peripheral vessels are reduced (Fig. 12.64). Blood flow in the contralateral lung is increased, and frequently this lung looks plethoric, an abnormality that may be more striking than the unilateral transradiance. The hilum of the involved lung is small but lung volumes are normal or only slightly decreased. The mediastinum may show some shift to the affected side at total lung capacity. 569 The fact that the ipsilateral lung volume is not increased is helpful in distinguishing Swyer–James syndrome from emphysema per se. 569 Ipsilateral air trapping is a key finding and a sine qua non of the condition. It can be demonstrated (Fig. 12.64) on an expiratory radiograph which should be exposed during a forced expiratory maneuver because the short expiratory time maximizes volume differences between the obstructed and nonobstructed lung. 581
CT shows changes that are often more complex than suspected from the chest radiograph (Fig. 12.65), and while it may confirm that the radiographic transradiancy is largely one-sided, it more commonly shows bilateral abnormalities.323.572. and 582. Areas of decreased attenuation on CT are often inhomogeneous, containing a patchwork of local decreased attenuation and hypovascular areas interspersed with lung of normal density. 572 Such small low-density areas may be poorly or sharply marginated, representing areas of small airways disease and air-trapping. 583 Air-trapping can be confirmed with expiratory CT scans. Other changes on CT include bronchiectasis, 323 which is a frequent, but not universal, finding,572. and 582. and areas of collapse and scarring.
The described combination of radiographic findings usually allows exclusion of other conditions that may resemble the Swyer–James syndrome. These conditions include congenital hypoplastic lung, congenital lobar emphysema, pulmonary artery hypoplasia, and proximal interruption of the pulmonary artery. The greatest concern is that signs of Swyer–James syndrome are being produced by a central, large airway obstruction, causing lung hypoventilation and a compensatory ipsilateral reduction in perfusion. This is a problem that may be resolved only by bronchoscopy or a tailored CT examination of the central airways.
In contrast to the apparently unilateral distribution of Swyer–James syndrome on chest radiography, CT usually reveals bilateral abnormalities (areas of decreased attenuation and cylindrical bronchiectasis) in individuals with the syndrome.
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
COPD (chronic airflow obstruction, chronic obstructive airway disease) encompasses a group of disorders characterized by chronic or recurrent obstruction to airflow.584. and 585. Historically, four principal disorders fall under this heading: asthma, chronic bronchitis, emphysema, and bronchiectasis.586. and 587. Although some purists object to the use of all-embracing, generic terms such as chronic obstructive airway disease or COPD, 588 they are necessary in clinical practice because the various forms often coexist to a variable extent in the same patient. 589 Nevertheless, the insight that some patients given the label of COPD have ‘pure’ bronchiectasis or emphysema has been provided by HRCT.590. and 591. In the round, COPD represents a considerable and underreported burden of morbidity across the world. 592 In industrialized and developing countries, cigarette smoking remains the most important risk factor for the development of COPD. 593
Asthma
Asthma is an inflammatory condition of the airways characterized by abnormal reactivity of the bronchi to various stimuli and by airflow obstruction that is at least partly reversible. This definition does not specify the degree of variation of airflow, but it is usually taken to be 15–20% of predicted values.
The pathologic changes of asthma have been studied largely,594. and 595. but not exclusively,596.597. and 598. by postmortem examination of lungs of patients who have died as a direct consequence of their asthma. Such findings represent the severe end of a spectrum of change. On gross examination the lungs are overinflated and fail to deflate because of tenacious mucus plugs in medium-sized airways. Bronchial mucosa is damaged or shed, and there are submucosal edema and inflammatory cell infiltrates of eosinophils, sometimes with lymphocytes and plasma cells, causing bronchial wall thickening. Other changes contributing to a general thickening of the bronchial walls include mucus gland hypertrophy, basement membrane thickening, and smooth muscle hyperplasia. 599
Pulmonary function tests are often normal in remission. In acute asthma the findings are increased airway resistance, increased total lung capacity (TLC), and increased residual volume and functional residual capacity (FRC) with decreased vital capacity (VC), indicating air trapping. 600 Diffusing capacity for carbon monoxide (DLco), particularly when corrected for accessible alveolar volume (Kco), is preserved in nonsmoking asthmatic people, even when the condition is severe. 601 Not surprisingly cigarette smoking adversely affects the pulmonary function profile of asthmatic individuals. 602
Chest radiography
Radiographic findings in simple asthma mirror the pathologic and functional changes of asthma. Hyperinflation may be seen in both relapse and remission. Hyperinflation is more frequent in patients with onset of asthma in the first or second decade than in those with a later onset. 603 Overinflation may be related to remodeling of the lungs and thoracic cage induced by airway obstruction during the growing phase. 604 Although less curved, the depressed hemidiaphragms rarely become flat or inverted in asthmatic individuals. The frequency of hyperinflation in adults with acute asthma has varied between approximately 20% and 70% in various series, reflecting different patient populations and hyperinflation criteria.294.605.606. and 607. In one series of 117 patients with a mean age of 41 years (range 13–75) admitted to hospital with acute asthma and in whom strict criteria for hyperinflation were applied, the prevalence was 39%. 606 While hyperinflation is often transient, lasting perhaps just 24 hours, 608 it may be a permanent change; in the study of 117 patients mentioned previously, 19% showed hyperinflation when in remission. 606 Hyperinflation in such patients is due to a change in lung compliance and is not caused by generalized emphysema, which can be shown to be absent by use of CT.601.603. and 609. However, if the patient is a smoker, emphysema may be a contributing factor.601. and 610.
In asthmatic patients the walls of end-on segmental airways may become conspicuously thickened (more than 1 mm) and normally invisible airways may be apparent as parallel or single-line opacities (Fig. 12.66). The most comprehensive radiographic study of bronchial wall thickening in asthma was that of Hodson and Trickey, 611 in which they assessed the finding on plain radiographs in 190 asthmatic patients ranging in age from 3 to 74 years. Bronchial wall thickening was found to be more frequent in children, and in the small number of children analysed it was a universal finding. Its frequency in adults was less but still surprisingly high; for example, 50% in the third and fourth decades. Other studies in adults also find that radiographic bronchial wall thickening is common: 71% in 48 asthmatic patients (nearly half of whom were smokers). 294 Bronchial wall thickening was 10 times more common in patients with an infective component to their asthma. In adults bronchial wall thickening, once developed, became a permanent feature. The irreversible nature of this bronchial wall thickening has also been shown on CT. 612
About 10% of asthmatic patients show prominence of the hila. This has been ascribed variously to nodal enlargement613 and vascular enlargement,606.614. and 615. in any event apparent hilar enlargement in asthmatic individuals without allergic bronchopulmonary aspergillosis (ABPA) is very unusual. The radiographic signs associated with asthma are summarized in Box 12.23.
Box 12.23
• Hyperinflation of the lungs (transient or fixed)
• Bronchial wall thickening
– Commoner in children
– Present in up to two-thirds of adult asthmatic individuals
– Usually an irreversible phenomenon
• Hilar prominence (uncommon)
A number of complications of asthma may be detected on the chest radiograph, 616 including consolidation, atelectasis, mucoid impaction, pneumothorax, pneumomediastinum, and allergic bronchopulmonary aspergillosis. Allergic aspergillosis apart, such complications are more common in children than in adults. In one series of 479 hospital patients with a median age of nearly 4 years, 22% had radiographic abnormalities, excluding signs of bronchial wall thickening and hyperinflation. 617 Other pediatric series bear these figures out.618. and 619. In adults the prevalence of similar abnormalities is generally less than 10%, and even in emergency admissions for acute asthma it is only about 1–2%.605.606.614. and 620. Higher figures are reported: in one series of patients with acute asthma, admitted after 12 hours of bronchodilator therapy in the emergency room, one-third of chest radiographs were abnormal. 607 A further large retrospective study of 1016 adults who were hospitalized with acute asthma showed that while just over half had normal chest radiographs on admission, 8.2% had significant complications of asthma on chest radiography (such as pneumothorax) and 6.7% had important incidental findings (for example, active tuberculosis). 621
Consolidation in asthma may be infective614 (Fig. 12.67) but it may also be due to eosinophilic consolidation, sometimes associated with allergic aspergillosis. Collapse (Fig. 12.67) ranges from subsegmental to lobar but occasionally involves a whole lung. 622 Such episodes of collapse are not necessarily associated with an acute illness, a respiratory tract infection, or a symptomatic worsening of the asthma.613. and 623. When segmental or lobar collapse occurs, the middle lobe is commonly affected both in children617 and in adults. 613 Collapse is due to mucoid impaction in large airways or, more commonly, mucus plugging in many small airways. The frequency of occurrence of collapse as such is difficult to determine because in many series differentiation between consolidation and collapse is not made. 606 In adults it is probably only a few percent.605. and 613.
Pneumothorax is an unusual complication of acute asthma in adults, and in a combination of historical series, largely of adults with acute disease,605.606.615. and 620. only three pneumothoraces in 566 patients were recorded. In a series reported by Burke, 624 it was noted that pneumothorax did not lead to death or morbidity and was usually not suspected clinically. A condition that may simulate tension pneumothorax in asthmatic patients receiving mechanical ventilation has been described; this is due to a ball valve mucus plug causing progressive localized or unilateral obstructive hyperinflation. 625
Pneumomediastinum in adults is as uncommon as pneumothorax, and in the combined series of 566 patients discussed previously, only two had a pneumomediastinum. Pneumomediastinum is considerably commoner in children (Fig. 12.67), with a prevalence of 5.4% in 515 acute asthma admissions. 617 In children it is much more common than pneumothorax, by a factor of 10 : 1 in one series. 626 Radiographically detectable complications in asthmatic patients, in approximate order of frequency, are listed in Box 12.24.
Box 12.24
• Complications of airway mucoid impaction
– Atelectasis (subsegmental through lobar)
– Consolidation (infective, eosinophilic, or ABPA)
• Features of allergic bronchopulmonary aspergillosis
– Proximal bronchiectasis
– Upper lobe fibrosis
• Pneumomediastinum or pneumothorax (both rare)
Although the indications for a chest radiograph in adults with asthma are not clear-cut, many authors recommend chest radiography in patients who are ill enough to justify admission to a hospital.605.606.607.620. and 627. In one series of 117 patients admitted to the hospital with acute asthma, 9% of chest radiographs showed abnormalities that altered management. 606 White and co-workers607 recorded an even higher figure, 22%, in 58 patients who were admitted after 12 hours of bronchodilator therapy in the emergency ward.
High-resolution computed tomography (Box 12.25)
Numerous CT studies have documented the sometimes subtle bronchial and parenchymal abnormalities that occur in asthmatic individuals.294.612.628.629.630.631.632.633.634. and 635. Dynamic studies, notably those of Brown et al.,636.637.638. and 639. have made effective use of the ability of HRCT to demonstrate changes in response to pharmacological agents.636.637.638.639.640.641.642.643.644.645. and 646. In between exacerbations, the airways and lung parenchyma and bronchi of individuals with early asthma appear normal, explaining the difficulty in discriminating between healthy individuals and people with mild asthma on the basis of HRCT appearances alone. 522
Box 12.25
HRCT findings in asthmatic individuals, in approximate order of prevalence (modified from Grenier et al. 631 with kind permission from Springer Science+Business Media)
• Bronchial wall thickening and narrowing of bronchial lumen
• Cylindrical bronchiectasis
• Thick linear opacities (atelectasis)
• Areas of decreased attenuation (part of mosaic pattern)
• Mucoid impaction in the large bronchi
• Small centrilobular opacities
• Airspace consolidation
• Thin-walled cysts (very rare)
HRCT shows abnormalities in between 68% and 90% of asthmatic patients.294.612. and 631. Principal abnormalities involved the airways (Fig. 12.68). Bronchial wall thickening was present in 16%, 612 82%, 631 and 92%294– a discrepancy that cannot be ascribed to the effects of smoking; although nearly half of the subjects in the study of Lynch et al. 294 were smokers, only 12 out of 50 of the asthmatic individuals in the study by Grenier et al. 631 were current or ex-smokers. Bronchial wall thickening is more prevalent in asthmatic patients with severe airflow obstruction (83% of patients with FEV1 <60% predicted, compared with 35% of patients with FEV1 >60% predicted). 630
Comparing bronchial dilatation in the series of Paganin and co-workers612 with that of Lynch and co-workers294 is difficult because patients with bronchiectasis (clinically and on CT criteria) were excluded from the latter study. 294 However, if cylindrical bronchiectasis and airway dilatation are taken as roughly equivalent terms, the findings are similar: cylindrical bronchiectasis in 56% of one series612 and bronchial dilatation in 77% of the other. 294 In the more recent study of Grenier et al. 631 frank bronchiectasis was identified in 29% of asthmatic patients, this lower prevalence perhaps reflecting more stringent criteria for the identification of bronchiectasis. The pathogenesis of these bronchial abnormalities remains controversial. Peribronchial inflammation may be partly responsible for the bronchial wall thickening but this feature is not always steroid-responsive. 612 Chronic inflammation of the airways probably leads to some morphologic remodeling with an increase in several components of the bronchial wall, including smooth muscle hypertrophy.647.648. and 649.
Explanations for the bronchial dilatation in asthmatic people, other than the obvious one of progressive damage by repeated episodes of inflammation, include coexisting subclinical allergic bronchopulmonary aspergillosis, 332 or the consequence of severe childhood infection. 650 However, no single explanation is entirely satisfactory, particularly as some reversibility of bronchiectatic changes is possible. 651 Furthermore, it seems surprising that, while there is no obvious relationship between the prevalence of bronchiectasis and the severity of asthma, 631 there is some correlation between bronchial wall thickness and the graded severity of asthma.598.633.644. and 652. So-called ‘objective’ CT measurement of bronchial dimensions, in particular wall thickness, is prone to many physiologic and technical vagaries, especially changes in window settings. 653 Nevertheless, comparative studies have lent some insights, such as the observation that wall thickening is a more pronounced feature in patients with nonallergic asthma, 629 although this difference has been questioned. 654
Small centrilobular opacities, prominent centrilobular structures, or the occasional tree-in-bud opacity are seen on HRCT in less than 20% in early studies of asthmatic patients294. and 631. and these changes likely reflect peribronchiolar inflammation and/or mucus stasis in small airways. A more recent study has highlighted the much higher prevalence, and irreversibility, of centrilobular opacities in patients with near-fatal asthma (100%) compared with moderate/severe asthma (70%) or mild asthma (36%). 655
The areas of decreased attenuation on HRCT that can be identified in acute or induced asthma almost certainly reflect hypoxic vasoconstriction in parts of the lung that are underventilated consequent upon the bronchospasm642. and 646. and such areas of air-trapping are more conspicuous and extensive on expiratory CT scans.508.513.630. and 656. In chronic asthma the significance of areas of ‘black lung’ on HRCT has in the past been controversial. Some studies have implied that emphysema is responsible for the areas of decreased lung attenuation612.629. and 632. in nonsmoking asthmatic people; against this is the striking lack of correlation between the Kco (the functional hallmark of emphysema) and the extent of areas of decreased attenuation in asthma601. and 657. and other airways diseases.438. and 658. There is increasing evidence in asthmatic individuals that morphologic features of emphysema on HRCT are almost invariably related to cigarette smoking, rather than the asthma per se in which the decreased attenuation merely represents small airways dysfunction.588.654.657.659.660. and 661. In patients with severe asthma a mosaic attenuation pattern, indistinguishable from that of patients with constrictive obliterative bronchiolitis, may be detectable on inspiratory HRCT. However, the decreased attenuation component of the mosaicism is usually not as extensive as that seen in individuals with clinically significant constrictive obliterative bronchiolitis. 521 A curiosity mentioned in a review of pathologic–HRCT findings in asthmatic individuals by Silva et al. 635 is a single report of a 27-year-old woman with scattered thin-walled cysts which the authors speculate were the consequence of bronchiolar obstruction.
Chronic bronchitis
Chronic bronchitis is clinically defined as a chronic or recurrent increase in the volume of mucoid bronchial secretions sufficient to cause expectoration and occurring on most days for 3 months in 2 or more successive years, other causes for expectoration having been excluded.203.586. and 662.
Chronic bronchitis is a common disease, affecting up to 20% of the adult population in some studies. 663 It seems to be much more common in males than females, 663 but when smoking habits are taken into account the difference is only 2 : 1. 587 Cigarette smoking is the most important factor associated with the development of chronic bronchitis. In eight combined series in England there was a sixfold rise in prevalence of chronic bronchitis, from 6.3% in nonsmokers to 40% in heavy smokers. 587 In epidemiologic studies a linear relationship exists between the amount smoked and the frequency of chronic bronchitis. 664 Less important factors include occupation, 665 environment, 666 age, and gender. 667 Given the subjective nature of the diagnosis, 668 it is not surprising that there appear to be substantial differences in the prevalence of chronic bronchitis even when there is no obvious difference in risk factors between cities in the same (Baltic) region. 669
The major pathologic changes are in the mucous glands, which show hypertrophy and hyperplasia and develop enlarged ducts. The enlargement of mucous glands can be quantified histologically using either the proportional mucous gland area or the Reid index, which is the ratio of gland thickness to bronchial wall thickness measured from epithelial basement membrane to perichondrium. 670 However, these indices do not clearly distinguish normal subjects from those with chronic bronchitis, since there is considerable overlap.671. and 672. Other pathologic changes in chronic bronchitis include goblet cell hyperplasia, squamous metaplasia of the epithelium, and a variable and often mild670 chronic inflammatory cell infiltrate. 203 Mucous plugs occur in the smaller airways, which themselves may be stenotic.587.673. and 674.
Chronic bronchitis typically occurs as a persistent productive cough following an acute chest infection. Such symptoms may persist for years with normal respiratory function tests and chest radiographs. Indeed the majority of such patients do not develop chronic airway obstruction, although they are at risk for recurrent episodes of purulent bronchitis. 675 In these patients the annual decrease in FEV1 is within the normal range,675. and 676. but, in heavy smokers, there is detectable airflow obstruction with a greater than predicted annual fall in FEV1. These patients become dyspneic, with copious sputum, and tend to become hypoxemic. 677 In such patients pulmonary arterial hypertension and cor pulmonale may develop. The majority of acute exacerbations in chronic bronchitis can be ascribed to a lower respiratory tract infection; 678 up to 30% are viral but other organisms, including atypical bacteria, such as Chlamydia pneumoniae, may occasionally be responsible. 679 However, the exact role of bacterial infections in exacerbations of COPD remains controversial. 680
Radiographic signs in ‘pure’ chronic bronchitis are poorly documented (Box 12.26). Nearly all the available information is derived from three series of patients, in whom there may have been coexistent emphysema.681.682. and 683. Overinflation of the lungs described in these reports is in conflict with the normal total lung capacity usually found in chronic bronchitis, 684 and can probably be ascribed to coexistent emphysema.
Box 12.26
• Chest radiograph usually normal
• Non-specific sign of bronchial wall thickening (ring and tramline shadows) and variably present ‘Increased lung markings’
• Signs of over-inflation likely reflect co-existing emphysema
• Enlargement of proximal pulmonary vessels and signs of cor pulmonale develop in hypoxic patients with advanced COPD
The majority of patients with symptoms of chronic bronchitis have a normal chest radiograph. 685 Radiographic signs of chronic bronchitis include bronchial wall thickening and ‘increased lung markings’. Bronchial wall thickening might be expected in chronic bronchitis because of the known pathologic changes in the airways. However, histologic studies suggest that the magnitude of these changes is small; in one report the absolute increase in gland thickness was only 0.1 mm. 672 Bates and co-workers681 described parallel line shadows on radiographs representing large airways seen side on (tramline opacities) in 42% of patients with chronic bronchitis. Ring shadows of approximately 6 mm in diameter adjacent to the upper poles of the hila represent the end-on anterior or posterior segmental airways of the upper lobes. This is a normal finding, whereas visible side-on airways (tramline opacities) are generally considered abnormal. Fraser and co-workers686 assessed the frequency of detection of end-on airways and their wall thickness in approximately 150 control subjects and 150 patients with chronic bronchitis. They found end-on airways visible in about 80% of both groups and a slight increase in wall thickness in chronic bronchitis, which was of limited value in distinguishing patients with chronic bronchitis from normal subjects (the sign was subject to significant interobserver variation). In functional terms, the ratio of wall thickness to external diameter in end-on airways does not appear to correlate with the degree of airflow obstruction. 687 Another radiographic sign described in the series of Bates and co-workers681 was increased lung markings, detected in 18%. The sign consists of small, ill-defined linear opacities with or without accentuation of small vascular opacities. 685 It is a subjective sign that pathologic examination, in one study, was shown to correlate with perivenous edema, inflammatory cell infiltration, and fibrosis. 688
Support for the finding of bronchial wall thickening in chronic bronchitis has come from HRCT studies300. and 560. in which proximal and distal bronchial wall thickening was present in 33% of smokers (compared with 18% of control subjects). 300 Contrary to expectation, HRCT has not fully elucidated the nature of the increased markings that constitute the ‘dirty lung’ of cigarette smokers, 689 but the spectrum of smoking-related parenchymal and airways is now better understood thanks largely to the investigations of Remy-Jardin.300.560. and 690. Air-trapping ascribable to airways (size unknown) dysfunction in cigarette smokers can be readily detected using expiratory HRCT, and can be demonstrated even in those individuals with normal pulmonary function test results.561. and 691. It has been shown that some parameters of histologic inflammation in cigarette smokers correlate with the degree of air-trapping as judged by attenuation of differences on inspiratory and expiratory HRCT in cigarette smokers. 692
Bronchial wall thickening cannot be regarded as a pathognomonic sign of smoking-related chronic bronchitis because it is nonspecific and is encountered in other inflammatory airway disease including asthma631 and infective or suppurative airways diseases, in particular bronchiectasis. 305 A study which evaluated wall thickness of airways in patients with chronic obstructive pulmonary disease showed that those patients with symptomatic chronic bronchitis had markedly thicker bronchial walls than those not fulfilling the clinical criteria for chronic bronchitis. 693 Automated software has shown that cigarette smokers with functional evidence of COPD have thicker airways than smokers without COPD or nonsmokers. 694 Quantitative CT studies have shown that bronchial wall thickening, over and above the extent of emphysema evident on CT, contributes to functional airflow limitation in cigarette smokers (Fig. 12.69).695. and 696.
Bronchial dilatation does not appear to be a clear-cut consequence of the bronchial wall inflammation associated with chronic bronchitis and, outside the ambit of age-related change298 and α1-antitrysin deficiency, 313 frank bronchiectasis does not seem to be a direct result of cigarette smoking. One of the original signs of chronic bronchitis, reported as a frequent finding in the era of bronchography, that is dilated mucous glands, seen as ‘bronchial pits’ on bronchograms, has been revisited by Zompatori et al., 697 who have pointed out that these small pits can be identified on CT along the inner surfaces of the large bronchi. These are shown to advantage on reformatted volumetric thin section CT (coronal reconstructions through the major airways) (Fig. 12.70). Whether this sign can be regarded as specific for chronic bronchitis (keeping in mind that Gamsu et al. 698 showed that such pits were relatively common in healthy adults on bronchography) is not known.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree