Fig. 23.1
Varying appearance of osteoid osteoma. OO tends to appear as a lytic focus with a central calcified nidus but the appearance varies. (a) Cortical, no sclerosis; (b) cortical, circumferential sclerosis; (c) cortical, asymmetric sclerosis; (d) biopsy-proven cortical OO with minimal lucency; (e) corticomedullary, circumferential sclerosis; (f) corticomedullary, asymmetric sclerosis; (g) medullary, no sclerosis; (h) medullary, circumferential sclerosis; (i) medullary, asymmetric sclerosis; (j) multifocal corticomedullary and medullary; (k) multifocal with two medullary foci (a third focus was present but not shown)
A Brodie’s abscess can mimic an osteoid osteoma. Misdiagnosis can lead to inadvertent treatment and procedural complications [10].
When a patient presents with a classic clinical history and stereotypical imaging findings, treatment can proceed in the absence of biopsy confirmation [11, 12]. When the clinical history or the imaging characteristics are in any way equivocal, a biopsy should be performed. Some operators routinely biopsy all lesions [13].
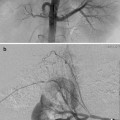
Osteoid osteomas spontaneously resolve over time but it can take several years [14–16]. Treatment options include conservative management, surgical resection or curettage, CT-guided resection (Fig. 23.2), and thermal ablation.
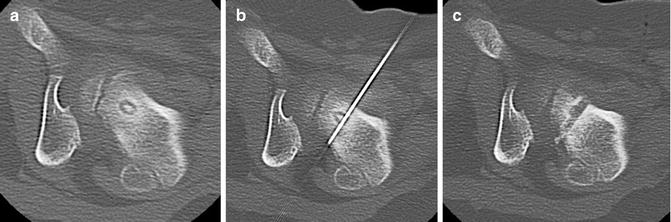

Fig. 23.2
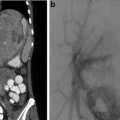
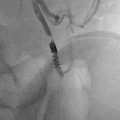
CT-guided excision. (a) Axial CT showing OO of the femoral neck. (b) A k-wire traverses the OO. (c) Residual tract after removal of a bone core. A second pass was used to remove the residual nidus. A k-wire is not necessary for OO excision; a large coring needle can be used instead
At the current time, thermal ablation of osteoid osteoma is the most common treatment approach. Heat applied to living cells results in cell damage or death dependent upon the temperature and the treatment time [17, 18]. Thermal therapy is most often performed using radiofrequency ablation (RFA) devices, but laser ablation has also been described [19–26]. There are single reports regarding treatment with coblation [27] and high-intensity focused ultrasound [28].
Indications/Contraindications
The most common indication for treatment is pain that is not responsive to NSAIDs. However, many families wish to undergo treatment to definitively treat a lesion and/or stop dependence on medication.
Equipment
Imaging
While fluoroscopy can be used to localize an OO, cross-sectional imaging provides more accurate information on the extent and shape of the lesion to aid in planning and localization. CT is currently used for initial assessment and localization of the lesion although MR guidance has been described [25, 26].
RFA
Application of radiofrequency energy results in frictional tissue heating. There are numerous radiofrequency devices on the market with varying monitoring approaches that include measuring resistance or temperature. For OO treatment, single-tine electrodes are often used, but a minimally deployed umbrella-shaped device can also be utilized. It is important to know the size and shape of the active heating zone of the specific device being used in order to create a treatment plan and assure safety of surrounding structures. The pattern of heat distribution tends to be ovoid for RFA.
Traditionally, monopolar devices have been used. They require grounding pads to complete the electrical circuit and provide heating. Proper placement of the pads (over muscle and without contact between pads) can be quite difficult in small children. Bipolar RFA devices that do not require the use of grounding pads have been introduced in recent years.
Laser
A diode laser can be used to perform thermal ablation of bone. Laser tuned to ~810 nm interact with hemoglobin to create heat. The heat is generated as a point source from the fiber tip. Laser systems are used to supply low power (2 W) setting in continuous mode. The total energy deposited determines the size of the area treated.
Bone Access
A standard bone access needle or a company-specific coaxial needle is used for access. Some needles are insulated to decrease heat transmission. For RFA the access needle is approximately 11–14 gauge. Laser requires smaller 14–18 gauge needles. Bone biopsy needles are used when required.
Preprocedure Workup
The patient’s imaging should be reviewed and further studies ordered if indicated. Blood work is not necessary in otherwise healthy patients.
A clinic visit is arranged to review the patient’s clinical history, discuss the treatment options (including conservative treatment), and obtain consent.
A combined approach with orthopedic surgery may be beneficial in patients with extremely thick, sclerotic overlying bone. An orthopedic bone drill can provide a large access route through the bone with minimal effort. Some access sets (Laurane, Bonopty) come with a manual drill that may also be helpful in this situation.
Procedure Technique
Treatment of OO causes significant pain so thermal ablation is performed under general anesthesia (even in adults). It is common to see an increase in heart rate and blood pressure in fully anesthetized patients when the nidus is accessed [29, 31]. Several procedural failures have been reported when ablation was attempted with sedation alone.
Antibiotic prophylaxis is used by some groups [12] but is not routinely administered.
Grounding pads are placed when necessary. The patient is positioned to allow access to the lesion keeping gantry size, instrumentation, and access during CT fluoroscopy in mind. CT is used to localize the lesion, mark the entry site, and plan the procedure.
It is essential to assure an approach that will minimize any potential risk of thermal injury to vital structures such as nerves, arteries, and skin; a distance of >1 cm should be sought [13, 29, 30]. When performing vertebral ablations, the perivenous plexus and cerebrospinal fluid can act as a heat sink. Thermal ablation in lesions with intact cortex is thought to be safe [32, 33] (Fig. 23.3) although risk of neural injury increases when using an RFA device with a 2 cm active zone [34]. When performing ablations in risky areas, the thermal protocol can be modified (less time, lower temperature, lower energy deposition) to improve safety.
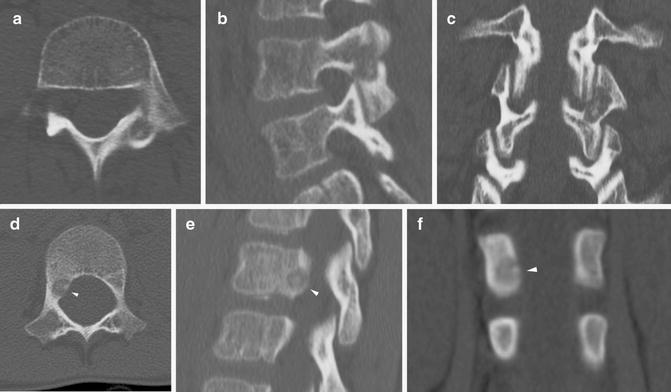

Fig. 23.3
Vertebral osteoid osteoma. (a–c) Axial, sagittal, and coronal images of OO of left L3 pars interarticularis and superior articular process. Intact cortex over the neural foramen protected against nerve injury. Some articular/cartilage damage was felt to be likely due to the lack of cortex in the area of the facet joint with thermal ablation, but the risk with surgical revision was felt to be greater. Laser ablation was undertaken without complication. (d–f) OO of right L1 pedicle (white arrowheads). The lesion abuts the dural sac and is within 2 mm of the exiting nerve. The lack of cortex medially raised significant concern of thermal injury to the L1 nerve. The patient’s family was given the option of ablation with saline infusion and temperature monitoring but declined intervention
In areas where heat spread could result in damage to surrounding structures, direct temperature monitoring with a thermocouple or injection of fluid or gas to act as a thermal barrier or heat sink should be considered. Fluid can be injected to protect joints, overlying subcutaneous tissues, or nearby vital structures (Fig. 23.4). Saline is not recommended when using RFA due to the potential to facilitate the RF signal due to the electrolyte content. In the spine, injection of saline, dextrose, contrast, or CO2 is used to provide protection in high-risk areas [35].

Fig. 23.4
Dextrose heat sink for RFA. (a, b) Axial and coronal CT demonstrate an OO adjacent to the femoral head. (c) Due to the location, a transcortical route along the femoral neck (to avoid entering the joint space) was not possible. (d) To minimize local heat damage to the joint capsule and cartilage, chilled dextrose was injected into the joint under ultrasound guidance. White arrowheads indicate the needle path. Yellow arrowheads outline dextrose beginning to distend the joint capsule
The area is prepped and draped. Fluoroscopy, CT, and/or CT fluoroscopy is used to guide insertion of the coaxial needle. A biopsy is performed when necessary. The RFA probe is then inserted and heating is commenced. If the coaxial needle is not insulated, it is pulled back over the RFA probe to decrease the chance of a tract/skin burn. The lesion is treated in accordance with the manufacturer’s instructions. Reported protocols vary with most in the range of 85–95 C × 4–8 min [12, 29].
There are a few differences when performing laser ablation. Prior to insertion of the coaxial bone access needle, the laser fiber is inserted and marked to show when 5 mm projects beyond the end of the coaxial needle (Fig. 23.5). Some operators use a second, smaller (18 gauge) needle to protect the laser fiber during insertion [24]. The laser fiber tip is charred in a small sample of the patient’s blood. In distinction to RFA, charring helps propagate laser energy. A biopsy sample is obtained to provide a path into the center of the lesion (so that the fragile glass fiber does not break during insertion). The generator is set to provide a power of 2 W in continuous mode and is activated for a specific time based on the total energy deposition desired to treat the lesion. Higher energy deposition results in a larger area (up to 1.6 cm) of ablation up to a maximum of approximately 1,000–1,200 J. When performing laser ablation in an area where there is the potential to damage surrounding structures, the desired power administered can be determined by the formula: (nidus size in mm × 100 J) + 200 J [24]. As the heat originates only from the tip, the fiber is placed centrally in the lesion. An ice pack is placed on the skin to protect against burns of the skin and tract from potential heat conduction through the uninsulated needle. For large lesions, the use of a beam splitter allows for simultaneous treatment of up to four sites.
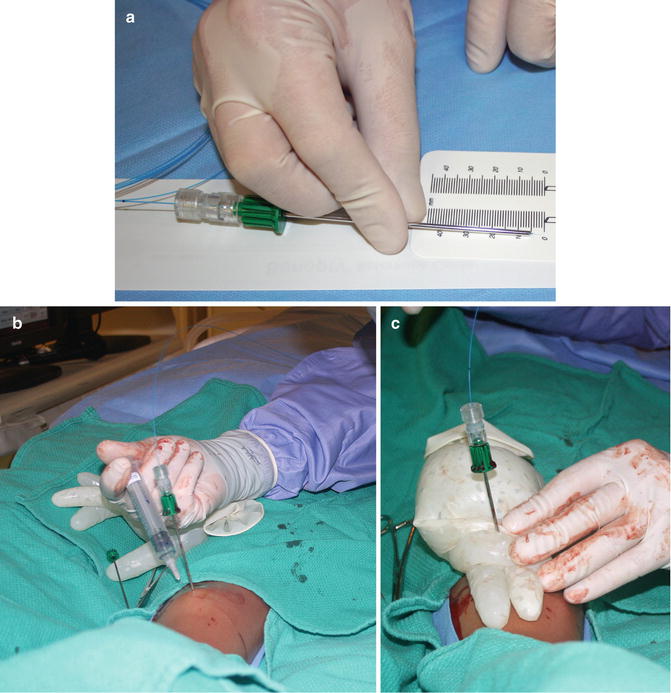

Fig. 23.5
Laser ablation. (a) The laser fiber (blue) is measured to assure that the tip projects 5 mm beyond the edge of the coaxial needle. In order to prevent a skin burn in case of heat transmission along the uninsulated coaxial needle, (b) saline and local anesthetic were used to “tent” the tissue overlying the tibia, and (c) ice was applied at the surface
Depending on the heat distribution characteristics of the modality used, complete treatment of larger lesions (>1 cm in any dimension) may require ablation in multiple locations. A report by Vanderschueren demonstrated that performing more than 1 burn cycle was the best predictor of successful RFA treatment, independent of lesion size [36].
Postprocedure Care
Immediately following the procedure, supportive care and analgesia is provided. An NSAID such as ketorolac will help decrease immediate postprocedure pain.
Following thermal ablation, some patients notice a change in the nature and/or intensity of pain immediately. In others, it can take 10–12 days.
Weight bearing is determined by the location of the intervention and the size and depth of the needle hole. For most patients with a low fracture risk, weight bearing as tolerated with no high-intensity exercise or contact sports for 4–6 weeks is an acceptable approach. In comparison, a patient who underwent an intervention in the intertrochanteric area with a long intraosseous pathway may require crutches with no weight bearing allowed for 6 weeks.
Informed discharge is performed. Signs and symptoms that would necessitate emergency treatment and a list of emergency contact numbers are discussed with the patient and their family. Ideally, a patient information sheet is given to the family.
Complications
Thermal, infectious, or lesion-related complications can occur.
Nearby structures such as arteries and nerves can be damaged during the heating process. Tracking of heat along the probe or guide needle could result in a burn to the needle tract or the skin [37]. Formation of a draining fistula has been reported following treatment of a tibial lesion [29].
Infectious complications include osteomyelitis, septic arthritis, and cellulitis.
Fracture can subsequently occur at the access site. The needle hole creates a riser concentrating stress in the area of the defect increasing the potential for fracture.
Incomplete or partial treatment can occur in approximately 10 % of patients necessitating a second treatment. Late recurrence of OO has been reported.
Follow-up
The patient is reassessed in the interventional radiology clinic 2 weeks following the procedure. If the patient has residual pain, a second procedure is booked.
There are no specific recommendations for imaging follow-up. While regular imaging can be performed to monitor the change in appearance of the OO, a change in the patient’s clinical status will provide an indication that repeat imaging should be undertaken.
Aneurysmal Bone Cyst
ABCs are benign multiloculated, cystic, expansile bone neoplasms that are locally aggressive [38–42]. Histologically, ABCs contain a fibroproliferative mesenchymal stroma with giant cell-like osteoclasts and vascular spaces [42]. The vascular spaces do not have an epithelial lining. The lytic lesions demonstrate a “soap bubble” appearance on plain films and commonly demonstrate fluid-fluid levels on CT and MRI. A rare solid variant exists. ABCs can occur in any bone but are most commonly seen in the femur, tibia, spine, and pelvis [43].
Telangiectatic osteosarcoma has a similar appearance to ABC creating a controversy surrounding the need for biopsy prior to treatment. Some authors feel that biopsy is absolutely essential to exclude telangiectatic osteosarcoma and will postpone treatment for several months [44–46]. Others perform biopsy at the time of treatment as long as no atypical or aggressive imaging or clinical findings are present [42, 47–50]. Both surgical and percutaneous biopsies have been advocated [45, 47, 49].
The pathogenesis of ABC has been controversial. Recently it was recognized as a clonal neoplasm driven by upregulation of the USP6 oncogene [38–41]. Historical hypotheses for formation include posttraumatic reaction, intraosseous vascular anomaly, and chromosomal abnormalities [45].
Several methods of categorization exist. If the ABC is an isolated finding, it is classified as primary. Secondary ABCs are associated with other osseous abnormalities (such as a giant cell tumor, chondroblastoma, fibrous dysplasia, osteoblastoma, and osteosarcoma).
A recent paper suggests classifying ABCs as lymphatic or venous anomalies based on the appearance of aspirated fluid contents. This differentiation predicts the likelihood of venous drainage (and consequent risk of sclerotherapy). Lambot-Juhan et al. demonstrated that venous drainage correlated with the appearance of fluid aspirated from ABCs. Cysts with sanguinous fluid all demonstrated venous drainage where only 1/7 did when the fluid was clear. Fluid that was intermediate in appearance showed venous drainage in 3 of 7 cases [46].
In addition to venous and lymphatic components, ABCs demonstrate variable arterial perfusion. Treatment-related deaths have been reported secondary to intraoperative bleeding in one patient [51] and retrograde arterial embolization of Ethibloc following treatment of a cervical ABC in another [52].
The lack of consensus in the literature makes specific treatment recommendations difficult. Surgical methods such as curettage or resection are associated with a recurrence rate as high as 71 % [42, 53]. Cryotherapy and radionuclide treatment have also been described [54, 55]. Interventional radiologists are involved when embolization or percutaneous sclerotherapy is undertaken.
Indications/Contraindications
ABC are routinely treated to stop potential expansion and instability of the involved osseous segment. The need for treatment is more urgent when there is a risk of fracture or the lesion threatens a growth plate or joint space. Nonaggressive lesions that do not threaten surrounding structures can be (closely) followed as spontaneous regression can occur [56–58].
There are no absolute contraindications to ABC treatment. Arterial embolization may not be possible in hypovascular lesions. Sclerosing agents can escape through draining veins resulting in systemic complications. When dealing with vertebral ABCs, some favor embolization as a primary treatment method [59, 60], and others specify that Ethibloc is contraindicated [45, 50].
Embolization
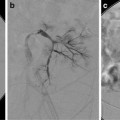
Embolization has been used both as a primary treatment method and as an adjunct to decrease blood loss prior to surgical intervention [59, 61, 62]. Arterial supply is variable with hypervascular, hypovascular, and combined perfusion patterns described.
Glue, polyvinyl alcohol (PVA) particles, and Gelfoam have been used as embolic agents. When the risks of nontarget embolization are low and deep penetration into small arteries is desired, 150–250 μm particles have been used. In the spinal area, where the risks of nontarget embolization are high, 250–350 μm particles have been used. Coils are not recommended in case repeat embolization is required.
When successful, symptoms resolved in days to weeks and ossification occurred within 2–4 months [59].
Sclerotherapy
Many interventional radiologists feel that sclerotherapy is the method of treatment of choice for most ABCs.
Numerous sclerosing agents have been used in the treatment of ABCs. The most common agents are described below.
Regardless of the agent used, sclerotherapy follows the basic approach described below. See Fig. 23.6.
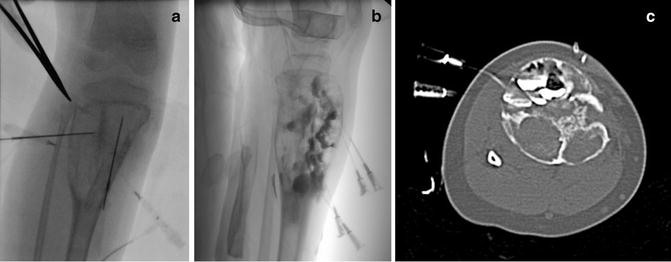

Fig. 23.6
Sclerosis of aneurysmal bone cyst. (a) Right tibial ABC prior to injection of sclerosing agent. (b) Lateral image demonstrates locules filled with doxycycline foam (that includes contrast). The tourniquet (seen at the top of the image) has been released. (c) CT imaging identified several posterior locules that have not yet been filled with sclerosant
1.
General anesthesia. Due to the pain associated with bone access and sclerosis, ABC treatment is performed under general anesthesia.
2.
Access lesion. Depending on the cortical thickness, a 12–16 gauge needle is introduced into the lesion using ultrasound, CT, or fluoroscopy. Biopsy specimens can be obtained at this time.
3.
Aspirate lesional fluid. The fluid is aspirated to assess appearance and volume.
4.
Inject contrast to determine volume, distribution pattern within locules, and the presence of venous drainage. Aspirate contrast when finished.
5.
Place secondary needles (if necessary). Multiple needles can be placed to maximize likelihood of treating entire lesion and/or to allow drainage of sclerosant.
(a)
Success treatment is dependent on sclerosant reaching every locule. This may be achieved by placement of multiple needles or repositioning of one needle in order to access all areas of the lesion.
(b)
A second needle is placed to allow drainage, decompression and a decrease in intralesional pressure (decreasing the risk of venous escape or extravasation of sclerosant).
6.
Apply tourniquet. Hand-tied tourniquets or orthopedic tourniquet machines can be used. The tourniquet pressure should not exceed systolic in order to maintain arterial inflow. A repeat contrast study is performed to assess for any change in vascular drainage. The tourniquets are left in place for approximately 10 min after sclerosis is finished to decrease risk of escape by allowing time for thrombosis and drainage from secondary needles to occur.
7.
Inject sclerosant. The sclerosant is injected slowly taking care that it fills the cavity gradually and does not leak out. The effluent from the other needle(s) is monitored for appearance. Depending on the location and sclerosant, the injection may be monitored during administration with ultrasound (especially doxycycline foam injection into solid tumor nodules), fluoroscopy, or CT. Cross-sectional imaging may be helpful in assuring that all locules are treated.
8.
Remove needles. Consider methods to decrease the risk of leakage of sclerosant as described below.
9.
Remove tourniquet. The tourniquet can be removed 5–10 min following completion of sclerosant administration. If the chance of venous escape of sclerosant is high, systemic effects can be seen with ethanol as a sclerosant. Gradually decreasing the pressure in an orthopedic tourniquet (e.g., 10 mmHg every 1–2 min) allows time for thrombosis and controlled release of any nontarget embolic.
10.
Follow–up imaging. The ABC is followed with serial radiographs (often at 1, 3, 6, and 12 months) and cross-sectional imaging (either MRI or CT).
11.
Repeat treatment as necessary. Repeat procedures may be necessary to completely treat locules not previously accessed or large or poorly responding lesions.
Doxycycline Foam +/− Tricalcium Phosphate Bone Graft
Doxycycline is tetracycline antibiotic that is effective as a direct tumor ablation agent. Tetracyclines have been associated with photosensitization and teeth yellowing in pediatric patients. Mild tooth discoloration from doxycycline has been seen rarely following large IV doses in children under 8 years of age. Discoloration can be treated with cleaning or bleaching. Photosensitivity has not been reported following doxycycline sclerosis in ABCs or lymphatic malformations.
The maximum dose for sclerosis has not been determined. A maximum dose of 300–500 mg in neonates and 1,000–1,200 mg in older patients has been suggested.
Doxycycline foam has been successfully used in the treatment of ABC [42]. Doxycycline injection treatment of ABCs can be routinely performed on an outpatient basis. The use of a bone graft substitute can increase effectiveness in larger locules.
Protein foam delays release of doxycycline for a longer antineoplastic effect, creates greater viscosity than a simple liquid embolic agent, and provides more contact between the doxycycline and cyst walls (tumoral elements).
To create 10 mg/mL doxycycline foam:
1.
Reconstitute doxycycline in normal saline or 0.5 % lidocaine with epinephrine (1:200,000) to create a 40 mg/mL solution.
2.
Combine:
(a)
5 mL of prepared doxycycline solution (200 mg)
(b)
5 mL of 25 % human serum albumin (if operators desire positive contrast in the foam, substitute 2.5 mL of water soluble contrast medium and 2.5 mL human serum albumin)
(c)
10 mL of air
3.
The mixture is then agitated with 2 syringes and a 3-way stopcock (at least 30 times) to produce the foam. Repeat agitation may be required due to settling throughout the procedure.
The foam is injected to fill the cyst or until the solution comes out through the other needle. Ultrasound can be used to inject the foam into solid ABC components.
When larger locules are present, a tricalcium phosphate bone graft substitute with doxycycline is subsequently used.
To create a Vitoss Flow (tricalcium phosphate) slurry:
1.
Break 5 mL block of Vitoss Foam Flow (Stryker Orthobiologics, Malvern, USA) into 1–2 mm fragments.
2.
Mix fragments with:
(a)
1 mL of contrast
(b)
4 mL of doxycycline/albumin solution (20 mg/mL)
3.
After sitting for at least a minute, pass the mixture back and forth between 2 syringes through a 3-way stopcock.
Cavities are filled to between 50 and 70 % of their volume leaving the second needle in place to clear the indwelling original doxycycline mixture. To minimize bleeding, Gelfoam pledgets can be inserted through needles as they are withdrawn.
Follow-up imaging with plain film is performed at 10-week intervals prior to subsequent treatment. Treatment is repeated every 12 weeks until full new bone healing and elimination of the cystic spaces or until residual lucent spaces show no evidence of further growth. Due to the slow growth of the ABC neoplastic cells, surveillance is maintained annually for 5 years in order to detect residual foci of ABC expansion that require focused percutaneous treatment.
Ethanol
The use of alcohol as a sclerosant for ABC was recently described [46]. Alcohol is a powerful sclerosant with a low viscosity that can cause significant morbidity or even mortality [63]. As a general rule, alcohol should only be administered by experienced operators utilizing meticulous technique.
When administering alcohol, use of the double (multiple) needle access technique is essential. Administering ethanol through one needle while simultaneously draining through another allows a greater volume of sclerosant to be delivered and decreases the intralesional pressure, making escape into venous structures less likely.
After accessing the lesion, anhydrous ethanol (96–98 % after exposure to air) is instilled to a maximum dose of 1 mL/kg. The tourniquet is deflated after 10 min. The patient is monitored in the hospital for 24 h.
Ethibloc
Ethibloc is a sclerosing agent available in Europe and Australia that includes alcohol, radiopaque contrast, and zein, a maize-derived protein [48–50, 64, 65]. When exposed to fluid, the viscous emulsion solidifies and the alcohol is slowly released.
When used for treatment of ABCs, it is injected through large-bore needles (14–18 gauge). A second needle is often used to allow evacuation of cyst contents or provide access to isolated locules. Depending on lesion size, up to 7.5 mL is injected. Some groups mix the 7.5 mL syringe of Ethibloc with an additional 1–5 mL of pure alcohol [48, 49]. Injections can be repeated every 1–2 months for large lesions that cannot be entirely treated with one procedure. The injection can be actively monitored with fluoroscopy or CT. Histacryl can be used to embolize the tract [66] or pressure is held for 10 min at the site [49].
Plain films and CT are obtained after the procedure. Patients are followed with plain films at 1, 3, 6, and 12 months and either CT or MRI at 6 and 12 months.
Local complications of Ethibloc injection include inflammation, sterile abscess formation, and soft tissue extrusion.
Pulmonary embolism and a high complication rate resulted in one group abandoning the use of Ethibloc [66]. However, the pulmonary embolism occurred following injection of 12 mL of Ethibloc into a pelvic ABC, 60 % more than the maximum volume of 7.5 mL recommended by the manufacturer [49].
As mentioned previously, death has been reported secondary to retrograde arterial embolization during treatment of a C2 ABC [52].
Injections
Steroid Injections
Background
Corticosteroids are a family of powerful anti-inflammatory medications. Corticosteroid injections are used in the treatment of juvenile arthritides and other inflammatory conditions. Injections can be performed to treat joints, tendon sheaths, bursae, neuromas, and ganglia or as a part of an invasive pain procedure, such as a celiac plexus block. This section will focus on joint and tendon injections.
In arthritis, steroid injections are often undertaken when oral anti-inflammatory medication does not provide adequate symptom relief.
Accurate delivery of corticosteroid into the affected area results in better symptom relief [67]. The accuracy of joint localization using clinical landmarks is as low as 50 %. Image guidance increases the accuracy of injection [67, 68]. This may be particularly helpful for complex or difficult-to-access joints such as the subtalar, sacroiliac, shoulder, and temporomandibular. While ultrasound is the most commonly used guidance modality, fluoroscopy, arthrography, CT,and MRI localization have all been described.
Contraindications
Contraindications to steroid administration include infectious arthritis, sepsis, joint instability, severe periarticular osteoporosis, and coagulopathy [69].
Equipment
Corticosteroids: Numerous corticosteroid preparations are commercially available. The dose, concentration, and duration vary by product. Steroids can be either soluble or insoluble. Insoluble corticosteroids form microcrystalline structures that vary in size and aggregation characteristics [70]. Soluble steroids, such as dexamethasone sodium phosphate, provide a rapid onset but a shorter duration in comparison with insoluble corticosteroids [69].
Preprocedure Workup
Patients are referred after assessment by a rheumatologist. Patients may require reassessment on the day of the procedure if there has been a recent change in their symptoms.
Review of the pertinent imaging is helpful. MRI can be used to identify the exact location of inflammation in areas of complex anatomy where localization through clinical examination is difficult (such as the feet).
Injections can be performed with local anesthesia only, sedation, or general anesthesia. The choice depends on the location and number of joints being injected and patient need.
No specific blood work or patient preparation is required.
Procedure
The patient is positioned to allow access to the targeted area(s). The appropriate imaging modality is used to introduce a small needle into the area to be injected. Depending on the anatomy of the joint, ultrasound, fluoroscopy, or CT are commonly used for access. Arthrography can be performed to assure an intra-articular location if desired (Fig. 23.7). When a joint effusion is present, fluid is collected to perform a cell count.
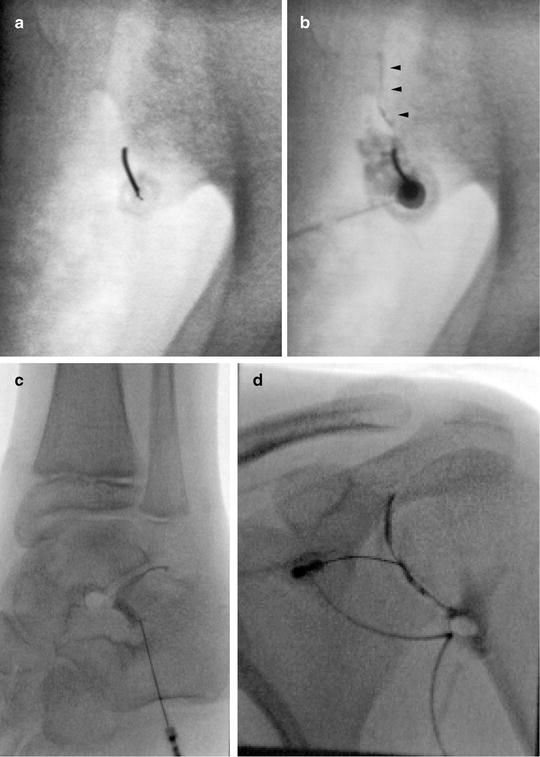

Fig. 23.7
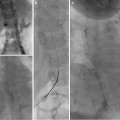
Arthrography. Arthrography can be used to assure the needle is in an intra-articular location prior to injection. (a) Fluoroscopy was used to insert needle into the lower portion of the sacroiliac joint. (b) Linear contrast outlines the SI joint (arrowheads) indicating an appropriate intra-articular position. The needle was repositioned after the initial contrast injection did not flow into the joint. CT guidance and arthrography are commonly used to access the SI joint. (c) Arthrogram of the posterior subtalar joint. (d) Shoulder arthogram
No scientifically determined dose parameters exist. As such, the dose is usually determined based on the product being used and institutional and personal preference by the rheumatologist and radiologist. Young et al. [71] recently proposed a dose protocol for triamcinolone acetonide and triamcinolone hexacetonide that is based on the patient age and the joint to be injected (Table 23.1). The doses in this study were calculated based on typical adult doses and scaled according to the body surface area based on weight/age. It should be noted that the radiologist may choose to decrease the prescribed dose/volume at the time of injection due to technical or anatomic concerns.
Table 23.1
Dose range for triamcinolone joint injections based on age in children
Location | Adult | 12 years old | 8 years old | 4 years old |
|---|---|---|---|---|
Knee | 20–40 | 15–30 | 10.6–21.2 | 7.8–15.6 |
Hip | ||||
Shoulder | ||||
Tibiotalar | 16–30 | 12–22.5 | 8.5–16 | 6.2–11.7 |
Elbow | 8–20 | 6–15 | 4.2–10.6 | 3.1–7.8 |
Radiocarpal | 8–20 | 6–15 | 4.2–10.6 | 3.1–7.8 |
Subtalar | 8–16 | 6–12 | 4.2–8.5 | 3.1–6.2 |
Cuboid-cuneiform | 8–14 | 6–10.5 | 4.2–7.4 | 3.1–5.5 |
Talonavicular | 8–12 | 6–9 | 4.2–6.4 | 3.1–4.7 |
Navicular-cuneiform | 8–12 | 6–9 | 4.2–6.4 | 3.1–4.7 |
Mid-cuneiform | 8 | 6 | 4.2 | 3.1 |
Intercarpal | 8 | 6 | 4.2 | 3.1 |
MCP/MTP | 6–8 | 4.5–6 | 3.2–4.2 | 2.3–3.1 |
PIP | 4–6 | 3–4.5 | 2.1–3.2 | 1.6–2.3 |
DIP | 2 | 1.5 | 1.1 | 0.8 |
Active monitoring during injection assures the needle tip is in an appropriate location and the steroid is injected into the targeted space/fluid without intra-pannus injection or extravasation (Fig. 23.8).
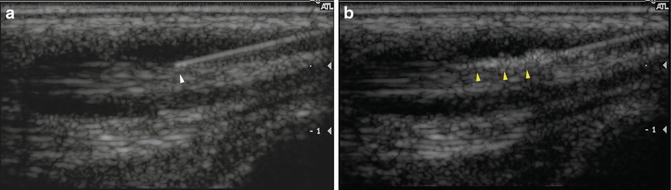

Fig. 23.8
Proper needle positioning. (a) Longitudinal ultrasound image shows the tip of a needle (white arrowhead) within a fluid-filled tendon sheath. (b) Ultrasound monitoring of the injection demonstrates corticosteroid flowing appropriately through the tendon sheath (yellow arrowheads). Active monitoring allows the injection to be halted and the needle repositioned if there is a problem
The corticosteroid can be mixed with local anesthetic or administered on its own and then followed by local anesthetic injection based on operator or institutional preference. It is thought that there may be less chance of leakage of corticosteroid into the surrounding tissues when it is “pushed in” with local anesthetic. However, when injecting a combined mixture, there is no need to change syringes leading to better needle stability.
Ultrasound examination at the time of procedure is also used to identify enlarged tendon sheaths that require injection. The steroid is injected into the tendon sheath taking care not to inject the tendon itself. Synechiae are sometimes identified at the time of injection (Fig. 23.9). There are anecdotal reports of attempted disruption of synechiae with a needle.
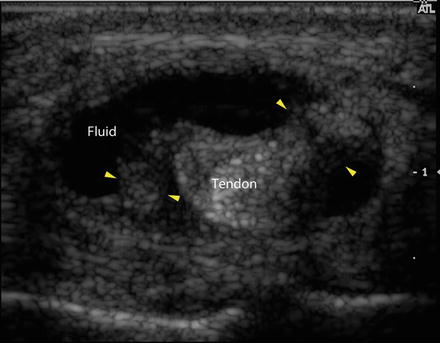

Fig. 23.9
Transverse ultrasound image demonstrates the central tendon surrounded by an enlarged, fluid-filled tendon sheath (labeled). Strands extending from the tendon to the edge of the tendon sheath (yellow arrowheads) are consistent with synechiae
When injections are being performed in areas where any possibility of arterial embolization carries high risk (e.g., spinal injections, especially transforaminal or intercostal nerve blocks), contrast injection with high-quality fluoroscopy or DSA should be used to monitor the injection [72].
Postprocedure Care
Patients may experience pain from the injection tract, from joint distension, or from the steroid itself. The latter is called postinjection or steroid flare and is thought to be secondary to the use of microcrystalline steroid preparations [69]. These causes of pain are self-limited and can be controlled with analgesics such as acetaminophen.
Patients and parents should be informed of the signs and symptoms of septic arthritis and given clear instructions to immediately contact their rheumatologist or the interventional radiology call service or go to the emergency room.