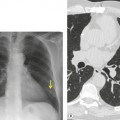
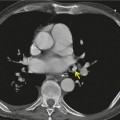
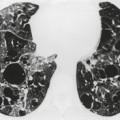
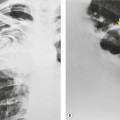
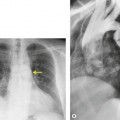
Numerous studies and clinical experience have convincingly shown the superiority of high-resolution computed tomography (HRCT) over chest radiography in terms of improved detection of diffuse lung disease, the provision of a histospecific diagnosis, and the assessment of disease reversibility. The development of HRCT has provided many insights about interstitial lung disease,1.2.3.4.5. and 6. large and small airways,7.8.9.10. and 11. and obstructive lung diseases.12. and 13. The technique is now mature but there are continuing minor modifications, such as volumetric rather than interspaced acquisition, 14 and these technical details are considered in Chapter 1.
NORMAL LUNG ANATOMY ON HRCT
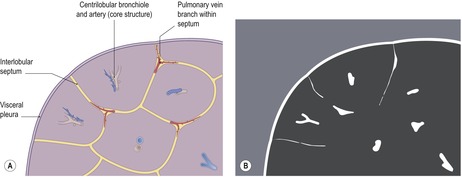
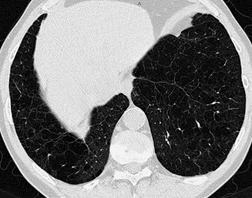
Before considering the individual HRCT patterns that reflect parenchymal and airways disease, an understanding of normal lung anatomy, with particular reference to the pulmonary lobule, is needed. The smallest objects that can be resolved on HRCT range from 100 µm to 400 µm and depend on the density, geometry, and orientation of the object in relation to the voxel. Accurate interpretation of HRCT of the diseased lung requires an appreciation of the appearances of normal structures, namely the bronchi, blood vessels, and the pulmonary lobule. The limits of spatial resolution determine the anatomy that can be identified on HRCT (Table 4.1).
| Normal lung structures visible on HRCT | Normal lung structures not visible on HRCT |
|---|---|
| Bronchi (down to eighth generation) | Lymphatic vessels |
| Pulmonary arteries | Alveoli and acini |
| Pulmonary veins | Capillary vessels |
| Interlobular septa (peripheral and occasional only) | Visceral pleura (nonfissural surface) |
| Visceral pleura (double layer as lobar fissures) | |
| Intrapulmonary lymph node (infrequent small nodule) |
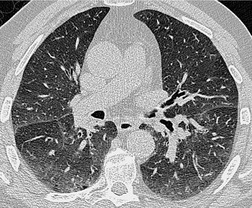
Throughout the lung the bronchi and pulmonary arteries run and branch together. Both the bronchi and pulmonary arteries taper slightly as they travel radially; this is most obvious in bronchi running parallel and within the plane of section. At any given level, the external diameter of the bronchus is almost exactly the same diameter as its accompanying pulmonary artery – the mean ratio of the external diameter of the pulmonary artery to the bronchus on HRCT has been reported to be 0.98 (standard deviation [SD] 0.14) but the range is wide (0.53–1.39). 15 The mean (SD) ratio of the internal luminal diameter of a bronchus to the diameter of its adjacent pulmonary artery for healthy individuals has been estimated to be 0.62 ± 0.13. 16 The bronchovascular bundle is surrounded by a connective tissue sheath from its origin at the hilum to the respiratory bronchioles in the lung periphery. The concept of separate, but connected, components making up the lung interstitium, propounded by Weibel, 17 is important to the understanding of HRCT findings in interstitial lung disease (the equivalent terms applicable to HRCT are given in parenthesis): the peripheral interstitium (subpleural interstitium) surrounds the surface of the lung beneath the visceral pleura and penetrates the lung to surround the pulmonary lobules (paraseptal interstitium). Within the lobules, a finer network of septal connective tissue fibers (intralobular interstitium) support the alveoli. The axial fibers form a sheath around the bronchovascular bundles (peribronchovascular interstitium) which extends from the pulmonary hilum to the lung periphery, as far out as the alveolar ducts and sacs. The connective tissue stroma of these separate components is in continuity and thus forms a fibrous skeleton for the lungs and a potential scaffold for diffuse infiltrative disease.
The interface between the bronchovascular bundle and surrounding lung on HRCT is clear-cut in the healthy state. Any thickening of the connective tissue interstitium will result in apparent bronchial wall thickening and some blurring of this interface. The size of the smallest subsegmental bronchi visible on HRCT is determined by the thickness of the bronchial wall rather than their diameter. In general, bronchi with a diameter of less than 3 mm with walls less than 300 µm thick are not identifiable on HRCT.18. and 19. Airways reach this critical size approximately 3 cm from the pleural surface.
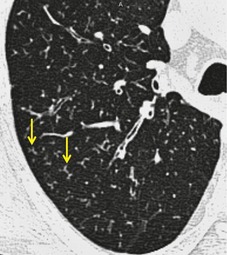
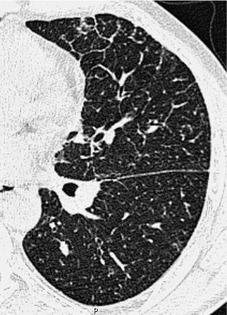
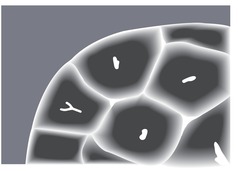
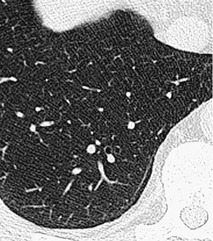
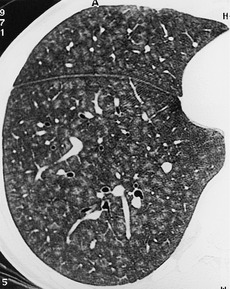
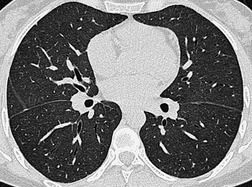
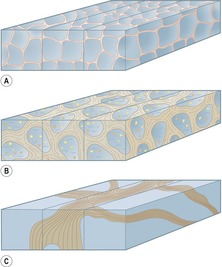
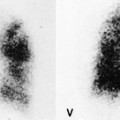
The pulmonary lobule is the smallest anatomic unit of the lung surrounded by a connective tissue septum, and in some ways the lobule resembles a lung in miniature20 (Fig. 4.1). Within the interlobular septa lie lymphatic channels and venules (Fig. 4.2). Abnormal thickening of the septa between the lobules is responsible for the short subpleural horizontal (Kerley B) lines seen on a chest radiograph. The lobule contains approximately 12 acini (but the range is wide, there may be between 3 and 25), each of which measures approximately 6–10 mm in diameter. Each lobule is about 2 cm in diameter and is polyhedral, sometimes resembling a truncated cone.21.22. and 23. A defining feature of the pulmonary lobule is the core structure – comprising the supplying bronchiole and the homologous pulmonary artery and lymphatics – which enters through the apex of the lobule. In the normal state the core structures, effectively the centrilobular artery (0.5–1 mm diameter) alone, are visible as dots 1 cm from the pleural surface. It is only when bronchioles become considerably thickened and surrounded by exudate that they become visible as V- or Y-shaped opacities (tree-in-bud pattern) (Fig. 4.3). The lung parenchyma between the core structures and interlobular septa is usually of homogeneous low attenuation, marginally greater than air.
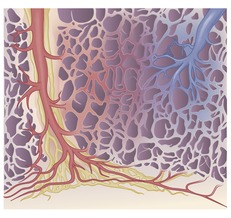 |
| Fig. 4.2 (Illustration by Aletta Ann Frazier, MD, Department of Radiologic Pathology, Armed Forces Institute of Pathology; redrawn with permission from Frazier AA et al. Pulmonary venoocclusive disease and pulmonary capillary hemangiomatosis. RadioGraphics 2007;27:877.) |
The connective tissue interlobular septa are well developed in the subpleural regions, particularly on the diaphragmatic surfaces and anterolateral regions of the lungs, where the bases of the cone-shaped lobules lie on the visceral pleural surface. In normal individuals, these interlobular septa measure approximately 100 µm in thickness. Because the lower limit of effective resolution of HRCT in vivo is approximately 200 µm they are rarely seen on HRCT. The few interlobular septa that are visible in healthy individuals are inconspicuous and are seen as straight lines 1–2 cm in length terminating at a visceral pleural surface. Sometimes several septa joining end to end are seen as a nonbranching linear structure measuring up to 4 cm; 18 these are most frequent at the lung bases overlying the diaphragmatic surface. Deep within the lung where the septa are less well developed, the interlobular septa are visible only when pathologically thickened.
By comparison with the epic work of Felson, 24 who documented the range of chest radiographic findings of healthy individuals (n=30 000), no similar HRCT study has been undertaken to establish the full spectrum of appearances of healthy individuals. As a consequence there may be difficulty in deciding whether subtle parenchymal perturbations on HRCT represent early (and significant) disease or are within the normal range. 25 An abnormality is usually regarded as synonymous with the presence of disease. However, HRCT findings considered abnormal in one group may not be of any clinical significance in another; for example, ill-defined centrilobular nodules and patchy ground-glass opacity seen on HRCT in a 50-year-old cigarette smoker may be considered to be within the range of expected findings, 26 whereas in a 25-year-old nonsmoker these same findings would be regarded as likely to represent early, and important, diffuse lung disease. In older individuals, HRCT findings of subpleural reticulation (presbyteric lung) and scattered thin-walled cystic airspaces as well as bronchial dilatation and wall thickening are surprisingly frequent and likely represent aging phenomena rather than important disease. 27
The close correlation between HRCT findings and macroscopic appearances of the lungs2.20. and 28. often allows accurate anatomic terms to be used in describing patterns of diffuse lung disease. Inexact terms, often used for the description of radiographs, have mostly been replaced by precise morphologic terms derived from an understanding of normal HRCT anatomy. Over the years there has been convergence on the majority of terms used in HRCT.29.30. and 31. Some of the most frequently encountered terms in the HRCT lexicon are included in the recently revised glossary of terms for thoracic imaging by the Fleischner Society32 and the illustrated glossary (which includes terms used for chest radiography) is provided here in its entirety for reference.
Fleischner Society:Glossary of Terms for Thoracic Imaging
The Fleischner glossary appears courtesy of RSNA. © RSNA, 2008. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL and Remy J. Fleischner Society: Glossary of Terms for Thoracic Imaging. Radiology 2008;246:697–722.
Members of the Fleischner Society compiled a glossary of terms for thoracic imaging that replaces previous glossaries published in 1984 and 1996 for thoracic radiography and computed tomography (CT), respectively. The need to update the previous versions came from the recognition that new words have emerged, others have become obsolete, the meaning of some terms has become obsolete, and the meaning of some terms has changed.
ACINUS
Anatomy. – The acinus is a structural unit of the lung distal to a terminal bronchiole and is supplied by first-order respiratory bronchioles; it contains alveolar ducts and alveoli. It is the largest unit in which all airways participate in gas exchange and is approximately 6–10 mm in diameter. One secondary pulmonary lobule contains between three and 25 acini. 20
Radiographs and CT. – Individual normal acini are not visible, but acinar arteries can occasionally be identified on thin-section CT scans. Accumulation of pathologic material in acini may be seen as poorly defined nodular opacities on chest radiographs and thin-section CT images. (See also nodule.)
ACUTE INTERSTITIAL PNEUMONIA, OR AIP
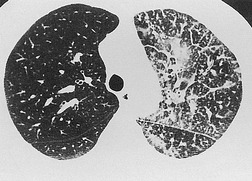
Pathology. – The term acute interstitial pneumonia is reserved for diffuse alveolar damage of unknown cause. The acute phase is characterized by edema and hyaline membrane formation. The later phase is characterized by airspace and/or interstitial organization. 33 The histologic pattern is indistinguishable from that of acute respiratory distress syndrome.
Radiographs and CT. – In the acute phase, patchy bilateral ground-glass opacities are seen, 34 often with some sparing of the individual lobules, producing a geographic appearance; dense opacification is seen in the dependent lung (Fig. 1). In the organizing phase, architectural distortion, traction bronchiectasis, cysts, and reticular opacities are seen. 35
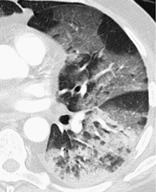 |
| Fig. 1 |
AIR BRONCHOGRAM
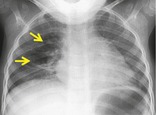
Radiographs and CT. – An air bronchogram is a pattern of air-filled (low-attenuation) bronchi (arrows) on a background of opaque (high-attenuation) airless lung (Fig. 2). The sign implies (a) patency of proximal airways and (b) evacuation of alveolar air by means of absorption (atelectasis) or replacement (e.g., pneumonia) or a combination of these processes. In rare cases, the displacement of air is the result of marked interstitial expansion (e.g., lymphoma). 36
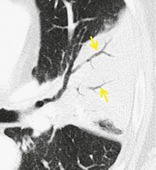 |
| Fig. 2 |
AIR CRESCENT
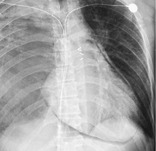
Radiographs and CT. – An air crescent is a collection of air in a crescentic shape (arrows) that separates the wall of a cavity from an inner mass (Fig. 3). The air crescent sign is often considered characteristic of either Aspergillus colonization of preexisting cavities or retraction of infarcted lung in angioinvasive aspergillosis.37. and 38. However, the air crescent sign has also been reported in other conditions, including tuberculosis, Wegener granulomatosis, intracavitary hemorrhage, and lung cancer. (See also mycetoma.)
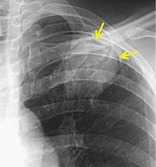 |
| Fig. 3 |
AIRSPACE
Anatomy. – An airspace is the gas-containing part of the lung, including the respiratory bronchioles but excluding purely conducting airways, such as terminal bronchioles.
Radiographs and CT. – This term is used in conjunction with consolidation, opacity, and nodules to designate the filling of airspaces with the products of disease. 42
AIR-TRAPPING
Pathophysiology. – Air-trapping is retention of air in the lung distal to an obstruction (usually partial).
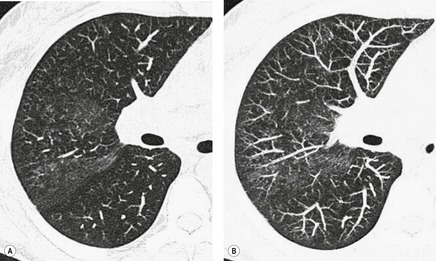
CT. – Air-trapping is seen on end-expiration CT scans as parenchymal areas with less than normal increase in attenuation and lack of volume reduction. Comparison between inspiratory and expiratory CT scans can be helpful when air-trapping is subtle or diffuse39. and 40. (Fig. 4). Differentiation from areas of decreased attenuation resulting from hypoperfusion as a consequence of an occlusive vascular disorder (e.g., chronic thromboembolism) may be problematic, 41 but other findings of airways versus vascular disease are usually present. (See also mosaic attenuation pattern.)
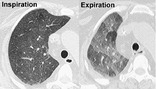 |
| Fig. 4 |
AORTOPULMONARY WINDOW
Anatomy. – The aortopulmonary window is the mediastinal region bounded anteriorly by the ascending aorta, posteriorly by the descending aorta, cranially by the aortic arch, inferiorly by the left pulmonary artery, medially by the ligamentum arteriosum, and laterally by the pleura and left lung.43. and 44.
Radiographs and CT. – Focal concavity in the left mediastinal border below the aorta and above the left pulmonary artery can be seen on a frontal radiograph (Fig. 5). Its appearance may be modified by tortuosity of the aorta. The aortopulmonary window is a common site of lymphadenopathy in a variety of inflammatory and neoplastic diseases.
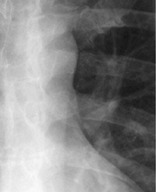 |
| Fig. 5 |
APICAL CAP
Pathology. – An apical cap is a caplike lesion at the lung apex, usually caused by intrapulmonary and pleural fibrosis pulling down extrapleural fat45 or possibly by chronic ischemia resulting in hyaline plaque formation on the visceral pleura. 46 The prevalence increases with age. It can also be seen in hematoma resulting from aortic rupture or in other fluid collection associated with infection or tumor, either outside the parietal pleura or loculated within the pleural space. 47
Radiographs and CT. – The usual appearance is of homogeneous soft tissue attenuation capping the extreme lung apex (uni- or bilaterally), with a sharp or irregular lower border (arrow, Fig. 6). Thickness is variable, ranging up to about 30 mm. 45 An apical cap occasionally mimics apical consolidation on transverse CT scans.
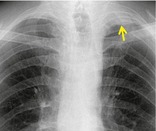 |
| Fig. 6 |
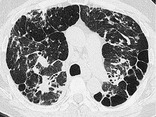
ARCHITECTURAL DISTORTION
Pathology. – Architectural distortion is characterized by abnormal displacement of bronchi, vessels, fissures, or septa caused by diffuse or localized lung disease, particularly interstitial fibrosis.
CT. – Lung anatomy has a distorted appearance and is usually associated with pulmonary fibrosis (Fig. 7) and accompanied by volume loss.
 |
| Fig. 7 |
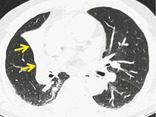
ATELECTASIS
Pathophysiology. – Atelectasis is reduced inflation of all or part of the lung. 48 One of the commonest mechanisms is resorption of air distal to airway obstruction (e.g., an endobronchial neoplasm). 49 The synonym collapse is often used interchangeably with atelectasis, particularly when it is severe or accompanied by obvious increase in lung opacity.
Radiographs and CT. – Reduced volume is seen, accompanied by increased opacity (chest radiograph) or attenuation (CT scan) in the affected part of the lung (arrows, Fig. 8). Atelectasis is often associated with abnormal displacement of fissures, bronchi, vessels, diaphragm, heart, or mediastinum. 50 The distribution can be lobar, segmental, or subsegmental. Atelectasis is often qualified by descriptors such as linear, discoid, or platelike. (See also linear atelectasis,rounded atelectasis.)
 |
| Fig. 8 |
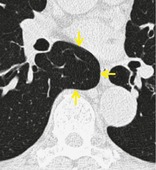
AZYGOESOPHAGEAL RECESS
Anatomy. – The azygoesophageal recess is a right posterior mediastinal recess into which the edge of the right lower lobe extends. It is limited superiorly by the azygos arch, posteriorly by the azygos vein and pleura anterior to the vertebral column, and medially by the esophagus and adjacent structures.
Radiographs and CT. – On a frontal chest radiograph, the recess is seen as a vertically oriented interface between the right lower lobe and the adjacent mediastinum (the medial limit of the recess). Superiorly, the interface is seen as a smooth arc with convexity to the left. Disappearance or distortion of part of the interface suggests disease (e.g., subcarinal lymphadenopathy). On CT scans, the recess (Fig. 9) merits attention because small lesions located in the recess will often be invisible on chest radiographs. 51
 |
| Fig. 9 |
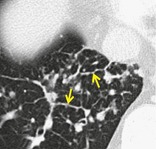
BEADED SEPTUM SIGN
CT. – This sign consists of irregular and nodular thickening of interlobular septa reminiscent of a row of beads (arrows, Fig. 10). It is frequently seen in lymphangitic spread of cancer and less often in sarcoidosis. 52
 |
| Fig. 10 |
BLEB
Anatomy. – A bleb is a small gas-containing space within the visceral pleura or in the subpleural lung, not larger than 1 cm in diameter. 53
CT. – A bleb appears as a thin-walled cystic airspace contiguous with the pleura. Because the arbitrary (size) distinction between a bleb and bulla is of little clinical importance, the use of this term by radiologists is discouraged.
BRONCHIECTASIS
Pathology. – Bronchiectasis is irreversible localized or diffuse bronchial dilatation, usually resulting from chronic infection, proximal airway obstruction, or congenital bronchial abnormality. 54 (See also traction bronchiectasis and traction bronchiolectasis.)
Radiographs and CT. – Morphologic criteria on thin-section CT scans include bronchial dilatation with respect to the accompanying pulmonary artery (signet ring sign), lack of tapering of bronchi, and identification of bronchi within 1 cm of the pleural surface55 (Fig. 11). Bronchiectasis may be classified as cylindric, varicose, or cystic, depending on the appearance of the affected bronchi. It is often accompanied by bronchial wall thickening, mucoid impaction, and small-airways abnormalities.14.55. and 56. (See also signet ring sign.)
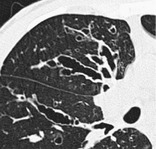 |
| Fig. 11 |
BRONCHIOLE
Anatomy. – Bronchioles are non-cartilage-containing airways. Terminal bronchioles are the most distal of the purely conducting airways; they give rise to respiratory bronchioles, from which the alveoli arise and permit gas exchange. Respiratory bronchioles branch into multiple alveolar ducts. 57
Radiographs and CT. – Bronchioles are not identifiable in healthy individuals, because the bronchiolar walls are too thin. 20 In inflammatory small-airways disease, however, thickened or plugged bronchioles may be seen as a nodular pattern on a chest radiograph or as a tree-in-bud pattern on CT scans.
BRONCHIOLECTASIS
Pathology. – Bronchiolectasis is defined as dilatation of bronchioles. It is caused by inflammatory airways disease (potentially reversible) or, more frequently, fibrosis.
CT. – When dilated bronchioles are filled with exudates and are thick walled, they are visible as a tree-in-bud pattern or as centrilobular nodules.11. and 58. In traction bronchiolectasis, the dilated bronchioles are seen as small, cystic, tubular airspaces (arrow), associated with CT findings of fibrosis (Fig. 12). (See also traction bronchiectasis and traction bronchiolectasis, tree-in-bud pattern.)
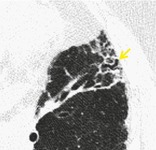 |
| Fig. 12 |
BRONCHIOLITIS
Pathology. – Bronchiolitis is bronchiolar inflammation of various causes. 59
CT. – This direct sign of bronchiolar inflammation (e.g., infectious cause) is most often seen as the tree-in-bud pattern, centrilobular nodules, and bronchiolar wall thickening on CT scans. (See also small-airways disease, tree-in-bud pattern.)
BRONCHOCELE
Pathology. – A bronchocele is bronchial dilatation due to retained secretions (mucoid impaction) usually caused by proximal obstruction, either congenital (e.g., bronchial atresia) or acquired (e.g., obstructing cancer). 60
Radiographs and CT. – A bronchocele is a tubular or branching Y- or V-shaped structure (arrow) that may resemble a gloved finger (Fig. 13). The CT attenuation of the mucus is generally that of soft tissue but may be modified by its composition (e.g., high-attenuation material in allergic bronchopulmonary aspergillosis). In the case of bronchial atresia, the surrounding lung may be of decreased attenuation because of reduced ventilation and, thus, perfusion.
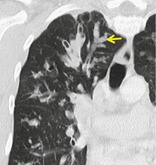 |
| Fig. 13 |
BRONCHOCENTRIC
CT. – This descriptor is applied to disease that is conspicuously centered on macroscopic bronchovascular bundles (Fig. 14). Examples of diseases with a bronchocentric distribution include sarcoidosis, 61 Kaposi sarcoma, 62 and organizing pneumonia. 63
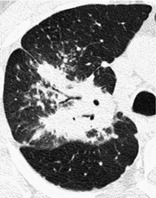 |
| Fig. 14 |
BRONCHOLITH
Pathology. – A broncholith, a calcified peribronchial lymph node that erodes into an adjacent bronchus, is most often the consequence of Histoplasma or tuberculous infection.
Radiographs and CT. – The imaging appearance is of a small calcific focus in or immediately adjacent to an airway (arrow, Fig. 15), most frequently the right middle lobe bronchus. Broncholiths are readily identified on CT scans. 64 Distal obstructive changes may include atelectasis, mucoid impaction, and bronchiectasis.
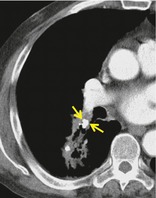 |
| Fig. 15 |
BULLA
Pathology. – An airspace measuring more than 1 cm – usually several centimeters – in diameter, sharply demarcated by a thin wall that is no greater than 1 mm in thickness. A bulla is usually accompanied by emphysematous changes in the adjacent lung. (See also bullous emphysema.)
Radiographs and CT. – A bulla appears as a rounded focal lucency or area of decreased attenuation, 1 cm or more in diameter, bounded by a thin wall (Fig. 16). Multiple bullae are often present and are associated with other signs of pulmonary emphysema (centrilobular and paraseptal).
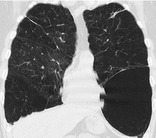 |
| Fig. 16 |
BULLOUS EMPHYSEMA
CAVITY
Radiographs and CT. – A cavity is a gas-filled space, seen as a lucency or low-attenuation area, within pulmonary consolidation, a mass, or a nodule (Fig. 17). In the case of cavitating consolidation, the original consolidation may resolve and leave only a thin wall. A cavity is usually produced by the expulsion or drainage of a necrotic part of the lesion via the bronchial tree. It sometimes contains a fluid level. Cavity is not a synonym for abscess.
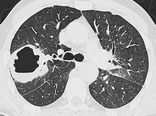 |
| Fig. 17 |
CENTRILOBULAR
Anatomy. – Centrilobular describes the region of the bronchiolovascular core of a secondary pulmonary lobule.19.20. and 65. This term is also used by pathologists to describe the location of lesions beyond the terminal bronchiole that center on respiratory bronchioles or even alveolar ducts.
CT. – A small dotlike or linear opacity in the center of a normal secondary pulmonary lobule, most obvious within 1 cm of a pleural surface, represents the intralobular artery (approximately 1 cm in diameter). 18 Centrilobular abnormalities include (a) nodules, (b) a tree-in-bud pattern indicating small-airways disease, (c) increased visibility of centrilobular structures due to thickening or infiltration of the adjacent interstitium, or (d) abnormal areas of low attenuation caused by centrilobular emphysema. 20 (See also lobular core structures.)
CENTRILOBULAR EMPHYSEMA
Pathology. – Centrilobular emphysema is characterized by destroyed centrilobular alveolar walls and enlargement of respiratory bronchioles and associated alveoli.66. and 67. This is the commonest form of emphysema in cigarette smokers.
CT. – CT findings are centrilobular areas of decreased attenuation, usually without visible walls, of nonuniform distribution and predominantly located in upper lung zones68 (Fig. 18). The term centriacinar emphysema is synonymous. (See also emphysema.)
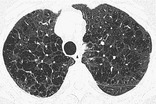 |
| Fig. 18 |
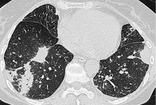
CONSOLIDATION
Pathology. – Consolidation refers to an exudate or other product of disease that replaces alveolar air, rendering the lung solid (as in infective pneumonia).
Radiographs and CT. – Consolidation appears as a homogeneous increase in pulmonary parenchymal attenuation that obscures the margins of vessels and airway walls69 (Fig. 19). An air bronchogram may be present. The attenuation characteristics of consolidated lung are only rarely helpful in differential diagnosis (e.g., decreased attenuation in lipoid pneumonia70 and increased in amiodarone toxicity). 71
 |
| Fig. 19 |
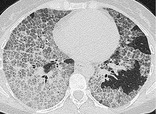
CRAZY-PAVING PATTERN
CT. – This pattern appears as thickened interlobular septa and intralobular lines superimposed on a background of ground-glass opacity (Fig. 20), resembling irregularly shaped paving stones. The crazy-paving pattern is often sharply demarcated from more normal lung and may have a geographic outline. It was originally reported in patients with alveolar proteinosis72 and is also encountered in other diffuse lung diseases73 that affect both the interstitial and airspace compartments, such as lipoid pneumonia. 74
 |
| Fig. 20 |
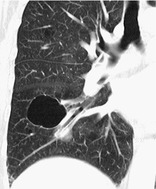
CYST
Pathology. – A cyst is any round circumscribed space that is surrounded by an epithelial or fibrous wall of variable thickness. 75
Radiographs and CT. – A cyst appears as a round parenchymal lucency or low-attenuating area with a well-defined interface with normal lung. Cysts have variable wall thickness but are usually thin-walled (<2 mm) and occur without associated pulmonary emphysema (Fig. 21). Cysts in the lung usually contain air but occasionally contain fluid or solid material. The term is often used to describe enlarged thin-walled airspaces in patients with lymphangioleiomyomatosis76 or Langerhans cell histiocytosis; 77 thicker walled honeycomb cysts are seen in patients with end-stage fibrosis. 78 (See also bleb,bulla,honeycombing,pneumatocele.)
 |
| Fig. 21 |
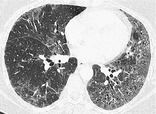
DESQUAMATIVE INTERSTITIAL PNEUMONIA, OR DIP
Pathology. – Histologically, DIP is characterized by the widespread accumulation of an excess of macrophages in the distal airspaces. The macrophages are uniformly distributed, unlike in respiratory bronchiolitis – interstitial lung disease, in which the disease is conspicuously bronchiolocentric. Interstitial involvement is minimal. Most cases of DIP are related to cigarette smoking, but a few are idiopathic or associated with rare inborn errors of metabolism. 33
Radiographs and CT. – Ground-glass opacity is the dominant abnormality and tends to have a basal and peripheral distribution (Fig. 22). Microcystic or honeycomb changes in the area of ground-glass opacity are seen in some cases. 79
 |
| Fig. 22 |
EMPHYSEMA
Pathology. – Emphysema is characterized by permanently enlarged airspaces distal to the terminal bronchiole with destruction of alveolar walls.66. and 67. Absence of ‘obvious fibrosis’ was historically regarded as an additional criterion, 66 but the validity of that criterion has been questioned because some interstitial fibrosis may be present in emphysema secondary to cigarette smoking.80. and 81. Emphysema is usually classified in terms of the part of the acinus predominantly affected: proximal (centriacinar, more commonly termed centrilobular, emphysema), distal (paraseptal emphysema), or whole acinus (panacinar or, less commonly, panlobular emphysema).
CT. – The CT appearance of emphysema consists of focal areas or regions of low attenuation, usually without visible walls. 82 In the case of panacinar emphysema, decreased attenuation is more diffuse. (See also bullous emphysema,centrilobular emphysema,panacinar emphysema,paraseptal emphysema.)
FISSURE
Anatomy. – A fissure is the infolding of visceral pleura that separates one lobe or part of a lobe from another; thus, the interlobar fissures are produced by two layers of visceral pleura. Supernumerary fissures usually separate segments rather than lobes. The azygos fissure, unlike the other fissures, is formed by two layers each of visceral and parietal pleura. All fissures (apart from the azygos fissure) may be incomplete.
Radiographs and CT. – Fissures appear as linear opacities, normally 1 mm or less in thickness, that correspond in position and extent to the anatomic fissural separation of pulmonary lobes or segments. Qualifiers include minor, major, horizontal, oblique, accessory, anomalous, azygos, and inferior accessory.
GROUND-GLASS OPACITY
Radiographs and CT. – On chest radiographs, ground-glass opacity appears as an area of hazy increased lung opacity, usually extensive, within which margins of pulmonary vessels may be indistinct. On CT scans, it appears as hazy increased opacity of lung, with preservation of bronchial and vascular margins (Fig. 23). It is caused by partial filling of airspaces, interstitial thickening (due to fluid, cells, and/or fibrosis), partial collapse of alveoli, increased capillary blood volume, or a combination of these, the common factor being the partial displacement of air.83. and 84. Ground-glass opacity is less opaque than consolidation, in which bronchovascular margins are obscured. (See also consolidation.)
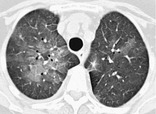 |
| Fig. 23 |
HALO SIGN
CT. – The halo sign is a CT finding of ground-glass opacity surrounding a nodule or mass (Fig. 24). It was first described as a sign of hemorrhage around foci of invasive aspergillosis. 85 The halo sign is nonspecific and may also be caused by hemorrhage associated with other types of nodules86 or by local pulmonary infiltration by neoplasm (e.g., adenocarcinoma). (See also reversed halo sign.)
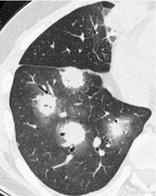 |
| Fig. 24 |
HILUM
Anatomy. – Hilum is a generic term that describes the indentation in the surface of an organ, where vessels and nerves connect with the organ. It is the site on the medial aspect of the lung where the vessels and bronchi enter and leave the lung.
Radiographs and CT. – A hilum appears as a composite opacity at the root of each lung produced by bronchi, arteries, veins, lymph nodes, nerves, and other tissue. The terms hilum (singular) and hila (plural) are preferred to hilus and hili respectively; the adjectival form is hilar.
HONEYCOMBING
Pathology. – Honeycombing represents destroyed and fibrotic lung tissue containing numerous cystic airspaces with thick fibrous walls, representing the late stage of various lung diseases, with complete loss of acinar architecture. The cysts range in size from a few millimeters to several centimeters in diameter, have variable wall thickness, and are lined by metaplastic bronchiolar epithelium. 75
Radiographs and CT. – On chest radiographs, honeycombing appears as closely approximated ring shadows, typically 3–10 mm in diameter with walls 1–3 mm in thickness, that resemble a honeycomb; the finding implies end-stage lung disease. On CT scans, the appearance is of clustered cystic airspaces, typically of comparable diameters of the order of 3–10 mm but occasionally as large as 2.5 cm (Fig. 25). Honeycombing is usually subpleural and is characterized by well-defined walls. 78 It is a CT feature of established pulmonary fibrosis. 33 Because honeycombing is often considered specific for pulmonary fibrosis and is an important criterion in the diagnosis of usual interstitial pneumonia, 87 the term should be used with care, as it may directly impact patient care.
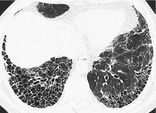 |
| Fig. 25 |
IDIOPATHIC PULMONARY FIBROSIS
Pathology. – Idiopathic pulmonary fibrosis is a specific form of chronic fibrosing interstitial pneumonia of unknown cause and is characterized by a histologic pattern of usual interstitial pneumonia.33. and 88.
Radiographs and CT. – The typical imaging findings are reticular opacities and honeycombing, with a predominantly peripheral and basal distribution (Fig. 26). Ground-glass opacity, if present, is less extensive than reticular and honeycombing patterns. The typical radiologic findings89. and 90. are also encountered in usual interstitial pneumonia secondary to specific causes, such as asbestos-induced pulmonary fibrosis (asbestosis), and the diagnosis is usually one of exclusion. (See also usual interstitial pneumonia.)
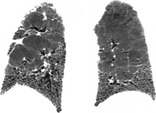 |
| Fig. 26 |
INFARCTION
Pathology. – Infarction is a process that may result in ischemic necrosis, usually the consequence of vascular compromise such as occlusion of a feeding pulmonary artery by an embolus (venous infarction is rare but recognized). Necrosis is relatively uncommon because tissue viability is maintained by the bronchial arterial blood supply. Pulmonary infarction may be secondary to a vasculitis (e.g., Wegener granulomatosis).
Radiographs and CT. – A pulmonary infarct is typically triangular or dome-shaped, with the base abutting the pleura and the apex directed toward the hilum (Fig. 27). The opacity represents local hemorrhage with or without central tissue necrosis.91. and 92.
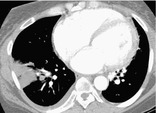 |
| Fig. 27 |
INFILTRATE
Radiographs and CT. – Formerly used as a term to describe a region of pulmonary opacification caused by airspace or interstitial disease seen on radiographs and CT scans. Infiltrate remains controversial because it means different things to different people. 93 The term is no longer recommended, and has been largely replaced by other descriptors. The term opacity, with relevant qualifiers, is preferred.
INTERLOBULAR SEPTAL THICKENING
Radiographs and CT. – This finding is seen on chest radiographs as thin linear opacities at right angles to and in contact with the lateral pleural surfaces near the lung bases (Kerley B lines); it is seen most frequently in lymphangitic spread of cancer or pulmonary edema. Kerley A lines are predominantly situated in the upper lobes, are 2–6 cm long, and can be seen as fine lines radially oriented toward the hila. In recent years, the anatomically descriptive terms septal lines and septal thickening have gained favor over Kerley lines. On CT scans, disease affecting one of the components of the septa (see interlobular septum) may be responsible for thickening and so render septa visible. On thin-section CT scans, septal thickening may be smooth or nodular94 (Fig. 28), which may help refine the differential diagnosis. (See also interlobular septum, beaded septum sign.)
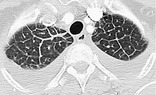 |
| Fig. 28 |
INTERLOBULAR SEPTUM
Anatomy. – Interlobular septa are sheetlike structures 10–20 mm long that form the borders of lobules; they are more or less perpendicular to the pleura in the periphery. Interlobular septa are composed of connective tissue and contain lymphatic vessels and pulmonary venules.
Radiographs and CT. – Interlobular septa appear as thin linear opacities between lobules (arrow, Fig. 29); these septa are to be distinguished from centrilobular structures. They are not usually seen in the healthy lung (normal septa are approximately 0.1 mm thick) but are clearly visible when thickened (e.g., by pulmonary edema). (See also interlobular septal thickening,lobule.)
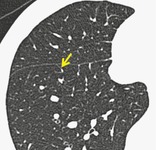 |
| Fig. 29 |
INTERSTITIAL EMPHYSEMA
Pathology. – Interstitial emphysema is characterized by air dissecting within the interstitium of the lung, typically in the peribronchovascular sheaths, interlobular septa, and visceral pleura. It is most commonly seen in neonates receiving mechanical ventilation.
Radiographs and CT. – Interstitial emphysema is rarely recognized radiographically in adults and is infrequently seen on CT scans (Fig. 30). It appears as perivascular lucent or low-attenuating halos (arrow) and small cysts.95. and 96.
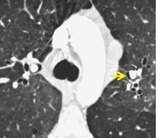 |
| Fig. 30 |
INTERSTITIUM
Anatomy. – The interstitium consists of a continuum of connective tissue throughout the lung comprising three subdivisions: (a) the bronchovascular (axial) interstitium, surrounding and supporting the bronchi, arteries, and veins from the hilum to the level of the respiratory bronchiole; (b) the parenchymal (acinar) interstitium, situated between alveolar and capillary basement membranes; and (c) the subpleural connective tissue contiguous with the interlobular septa. 17
INTRALOBULAR LINES
CT. – Intralobular lines are visible as fine linear opacities in a lobule when the intralobular interstitial tissue is abnormally thickened (Fig. 31). When numerous, they may appear as a fine reticular pattern. Intralobular lines may be seen in various conditions, including interstitial fibrosis and alveolar proteinosis. 18
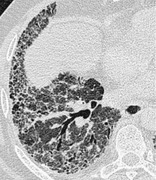 |
| Fig. 31 |
JUXTAPHRENIC PEAK
Radiographs and CT. – A juxtaphrenic peak is a small triangular opacity based at the apex of the dome of a hemidiaphragm, associated with upper lobe volume loss of any cause (e.g., postirradiation fibrosis or upper lobectomy). 97 It is most readily appreciated on a frontal chest radiograph (Fig. 32). The peak is caused by upward retraction of the inferior accessory fissure98 or an intrapulmonary septum associated with the pulmonary ligament. 99
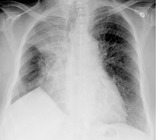 |
| Fig. 32 |
LINEAR ATELECTASIS
Radiographs and CT. – Linear atelectasis is a focal area of subsegmental atelectasis with a linear configuration, almost always extending to the pleura. 100 It is commonly horizontal but sometimes oblique or vertical. The thickness of the atelectasis may range from a few millimeters to more than 1 cm (Fig. 33). Linear atelectasis is also referred to as discoid or platelike atelectasis.100 (See also atelectasis.)
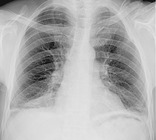 |
| Fig. 33 |
LOBE
Anatomy. – The lobe is the principal division of the lungs (normally, three lobes on the right and two on the left); each lobe is enveloped by visceral pleura, except at the lung root (hilum) and when an interlobar fissure is incomplete.
LOBULAR CORE STRUCTURES
Anatomy. – Lobular core structures are the central structures in secondary pulmonary lobules and consist of a centrilobular artery and bronchiole. 65
CT. – The pulmonary artery and its immediate branches are visible in the center of a secondary lobule on thin-section CT scans, particularly if thickened (e.g., by pulmonary edema) (arrow, Fig. 34). These arteries measure approximately 0.5–1.0 mm in diameter. However, the normal bronchiole in the center of the secondary pulmonary lobule cannot be seen on thin-section CT scans because of the thinness of its wall (approximately 0.15 mm).18. and 20. (See also centrilobular,lobule.)
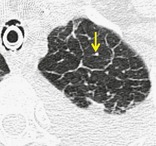 |
| Fig. 34 |
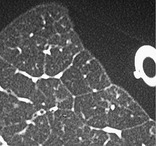
LOBULE
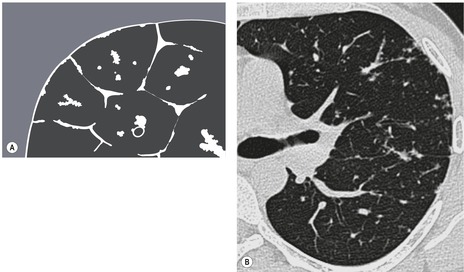
Anatomy. – The lobule is the smallest unit of lung surrounded by connective-tissue septa, as defined by Miller101 and Heitzman et al. 65 The lobule is also referred to as the secondary pulmonary lobule; it contains a variable number of acini, is irregularly polyhedral in shape, and varies in size from 1.0 to 2.5 cm in diameter. The centrilobular structures, or core structures, include bronchioles and their accompanying pulmonary arterioles and lymphatic vessels. The connective-tissue septa surrounding the pulmonary lobule – the interlobular septa, which contain veins and lymphatic vessels – are best developed in the periphery in the anterior, lateral, and juxtamediastinal regions of the upper and middle lobes.
CT. – On thin-section CT scans, the three basic components of the lobule – the interlobular septa and septal structures, the central lobular region (centrilobular structures), and the lobular parenchyma – can be identified, particularly in disease states. Peripheral lobules are more uniform in appearance and pyramidal in shape than are central lobules20 (Fig. 35). (See also interlobular septum,lobular core structures.)
 |
| Fig. 35 |
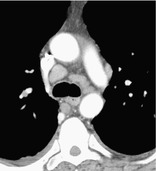
LYMPHADENOPATHY
Pathology. – By common usage, the term lymphadenopathy is usually restricted to enlargement, due to any cause, of the lymph nodes. Synonyms include lymph node enlargement (preferred) and adenopathy.
CT. – There is a wide range in the size of normal lymph nodes. Mediastinal and hilar lymph nodes range in size from sub-CT resolution to 12 mm. Somewhat arbitrary thresholds for the upper limit of normal of 1 cm in short-axis diameter for mediastinal nodes102 and 3 mm for most hilar nodes103 have been reported, but size criteria do not allow reliable differentiation between healthy and diseased lymph nodes (Fig. 36).
 |
| Fig. 36 |
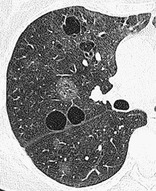
LYMPHOID INTERSTITIAL PNEUMONIA, OR LIP
Pathology. – LIP is a rare disease characterized by diffuse pulmonary lymphoid proliferation with predominant interstitial involvement. It is included in the spectrum of interstitial pneumonias and is distinct from diffuse lymphomas of the lung. Features include diffuse hyperplasia of bronchus-associated lymphoid tissue and diffuse polyclonal lymphoid cell infiltrates surrounding the airways and expanding the lung interstitium. LIP is usually associated with autoimmune diseases or human immunodeficiency virus infection.33. and 104.
CT. – Ground-glass opacity is the dominant abnormality, and thin-walled perivascular cysts may be present (Fig. 37). Lung nodules, a reticular pattern, interlobular septal and bronchovascular thickening, and widespread consolidation may also occur.105. and 106.
 |
| Fig. 37 |
MASS
Radiographs and CT. – A mass is any pulmonary, pleural, or mediastinal lesion seen on chest radiographs as an opacity greater than 3 cm in diameter (without regard to contour, border, or density characteristics). Mass usually implies a solid or partly solid opacity. CT allows more exact evaluation of size, location, attenuation, and other features. (See also nodule.)
MEDIASTINAL COMPARTMENTS
Anatomy. – Nominal anatomic compartments of the mediastinum include the anterior, middle, posterior, and (in some schemes) superior compartments. The anterior compartment is bounded anteriorly by the sternum and posteriorly by the anterior surface of the pericardium, the ascending aorta, and the brachiocephalic vessels. The middle compartment is bounded by the posterior margin of the anterior division and the anterior margin of the posterior division. The posterior compartment is bounded anteriorly by the posterior margins of the pericardium and great vessels and posteriorly by the thoracic vertebral bodies. In the four-compartment model, the superior compartment is defined as the compartment above the plane between the sternal angle to the T4–5 intervertebral disk or, more simply, above the aortic arch.107. and 108. Exact anatomic boundaries between the compartments do not exist, and there are no barriers (other than the pericardium) to prevent the spread of disease between compartments. Other classifications exist, but the three- and four-compartment models are the most commonly used.
MICRONODULE
CT. – A micronodule is a discrete, small, round, focal opacity. A variety of diameters have been used in the past to define a micronodule; for example, a diameter of no greater than 7 mm. 109 Use of the term is most often limited to nodules with a diameter of less than 5 mm110 or less than 3 mm. 111 It is recommended that the term be reserved for opacities less than 3 mm in diameter. (See also nodule,miliary pattern.)
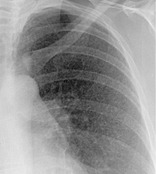
MILIARY PATTERN
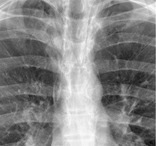
Radiographs and CT. – On chest radiographs, the miliary pattern consists of profuse tiny, discrete, rounded pulmonary opacities (≤3 mm in diameter) that are generally uniform in size and diffusely distributed throughout the lungs (Fig. 38). This pattern is a manifestation of hematogenous spread of tuberculosis and metastatic disease. Thin-section CT scans show widespread, randomly distributed micronodules.
 |
| Fig. 38 |
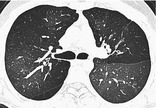
MOSAIC ATTENUATION PATTERN
CT. – This pattern appears as a patchwork of regions of differing attenuation that may represent (a) patchy interstitial disease, (b) obliterative small-airways disease (Fig. 39), or (c) occlusive vascular disease. 112Mosaic attenuation pattern is a more inclusive term than the original terms mosaic oligemia and perfusion.113 Air-trapping secondary to bronchial or bronchiolar obstruction may produce focal zones of decreased attenuation, an appearance that can be enhanced by using expiratory CT.114. and 115. The mosaic attenuation pattern can also be produced by interstitial lung disease characterized by ground-glass opacity; in this situation, areas of higher attenuation represent the interstitial process and areas of lower attenuation represent the normal lung.
 |
| Fig. 39 |
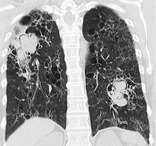
MYCETOMA
Pathology. – A mycetoma is a discrete mass of intertwined hyphae, usually of an Aspergillus species, matted together by mucus, fibrin, and cellular debris colonizing a cavity, usually from prior fibrocavitary disease (e.g., tuberculosis or sarcoidosis).
Radiographs and CT. – A mycetoma may move to a dependent location when the patient changes position and may show an air crescent sign (Fig. 40). CT scans may show a spongelike pattern and foci of calcification in the mycetoma. 116 A synonym is fungus ball. (See also air crescent.)
 |
| Fig. 40 |
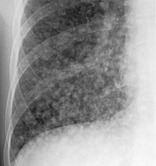
NODULAR PATTERN
Radiographs and CT. – A nodular pattern is characterized on chest radiographs by the presence of innumerable small rounded opacities that are discrete and range in diameter from 2 to 10 mm (Fig. 41). The distribution is widespread but not necessarily uniform. On CT scans, the pattern may be classified as one of three anatomic distributions: centrilobular, lymphatic, or random. (See also nodule.)
 |
| Fig. 41 |
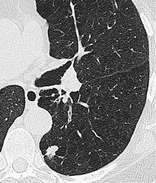
NODULE
Radiographs and CT. – The chest radiographic appearance of a nodule is a rounded opacity, well or poorly defined, measuring up to 3 cm in diameter. (a) Acinar nodules are round or ovoid poorly defined pulmonary opacities approximately 5–8 mm in diameter, presumed to represent an atomic acinus rendered opaque by consolidation. This classification is used only in the presence of numerous such opacities. (b) A pseudonodule mimics a pulmonary nodule; it represents, for example, a rib fracture, a skin lesion, a device on the surface of the patient, anatomic variants, or composite areas of increased opacity. 117
On CT scans, a nodule appears as a rounded or irregular opacity, well or poorly defined, measuring up to 3 cm in diameter (Fig. 42). (a) Centrilobular nodules appear separated by several millimeters from the pleural surfaces, fissures, and interlobular septa. They may be of soft tissue or ground-glass attenuation. Ranging in size from a few millimeters to a centimeter, centrilobular nodules are usually ill-defined. 20(b) A micronodule is less than 3 mm in diameter (see also micronodule). (c) A ground-glass nodule (synonym, non-solid nodule) manifests as hazy increased attenuation in the lung that does not obliterate the bronchial and vascular margins. (d) A solid nodule has homogeneous soft tissue attenuation. (e) A part-solid nodule (synonym, semi-solid nodule) consists of both ground-glass and solid soft tissue attenuation components. (See also mass.)
 |
| Fig. 42 |
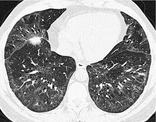
NONSPECIFIC INTERSTITIAL PNEUMONIA, OR NSIP
Pathology. – NSIP is characterized by a histologic pattern of uniform interstitial involvement by varying degrees of chronic inflammation or fibrosis. NSIP may be idiopathic or seen in other settings, including collagen vascular disease, hypersensitivity pneumonitis, drug-induced lung disease, infection, and immunodeficiency (including human immunodeficiency virus infection). 33
CT. – NSIP has variable thin-section CT appearances: the most frequent is ground-glass opacities with reticulation, traction bronchiectasis or bronchiolectasis, and little or no honeycombing (Fig. 43). The distribution is usually basal and subpleural. 118
 |
| Fig. 43 |
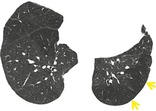
OLIGEMIA
Pathophysiology. – Oligemia is a reduction in pulmonary blood volume. Most frequently, this reduction is regional, but occasionally it is generalized. Regional oligemia is usually associated with reduced blood flow in the oligemic area.
Radiographs and CT. – Oligemia appears as a regional or widespread decrease in the size and number of identifiable pulmonary vessels (arrows, Fig. 44), which is indicative of less than normal blood flow. (See also mosaic attenuation pattern,pulmonary blood flow redistribution.)
 |
| Fig. 44 |
OPACITY
Radiographs and CT. – Opacity refers to any area that preferentially attenuates the X-ray beam and therefore appears more opaque than the surrounding area. It is a nonspecific term that does not indicate the size or pathologic nature of the abnormality. (See also parenchymal opacification,ground-glass opacity.)
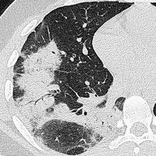
ORGANIZING PNEUMONIA
Pathology. – Organizing pneumonia manifests as a histologic pattern characterized by loose plugs of connective tissue in the airspaces and distal airways. Interstitial inflammation and fibrosis are minimal or absent. Cryptogenic organizing pneumonia, or COP, is a distinctive clinical disorder among the idiopathic interstitial pneumonias, 33 but the histologic pattern of organizing pneumonia is encountered in many different situations, including pulmonary infection, hypersensitivity pneumonitis, and collagen vascular diseases.
Radiographs and CT. – Airspace consolidation is the cardinal feature of organizing pneumonia on chest radiographs and CT scans. In COP, the distribution is typically subpleural and basal (Fig. 45) and sometimes bronchocentric. 119 Other manifestations of organizing pneumonia include ground-glass opacity, tree-in-bud pattern, and nodular opacities. 63
 |
| Fig. 45 |
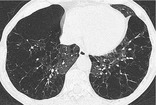
PANACINAR EMPHYSEMA
Pathology. – Panacinar emphysema involves all portions of the acinus and secondary pulmonary lobule more or less uniformly. 66 It predominates in the lower lobes and is the form of emphysema associated with α1-antitrypsin deficiency.
CT. – Panacinar emphysema manifests as a generalized decrease of the lung parenchyma attenuation with a decrease in the caliber of blood vessels in the affected lung120. and 121. (Fig. 46). Severe panacinar emphysema may coexist and merge with severe centrilobular emphysema. The appearance of featureless decreased attenuation may be indistinguishable from severe constrictive obliterative bronchiolitis. 13 The term panlobular emphysema is synonymous. (See also emphysema.)
 |
| Fig. 46 |
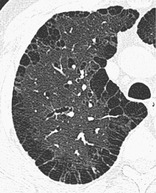
PARASEPTAL EMPHYSEMA
Pathology. – Paraseptal emphysema is characterized by predominant involvement of the distal alveoli and their ducts and sacs. It is characteristically bounded by any pleural surface and the interlobular septa.66. and 67.
CT. – This emphysema is characterized by subpleural and peribronchovascular regions of low attenuation separated by intact interlobular septa (Fig. 47), sometimes associated with bullae. The term distal acinar emphysema is synonymous. (See also emphysema.)
 |
| Fig. 47 |
PARENCHYMA
Anatomy. – Parenchyma refers to the gas-exchanging part of the lung, consisting of the alveoli and their capillaries.
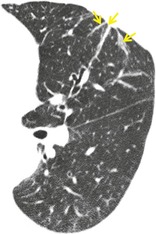
PARENCHYMAL BAND
Radiographs and CT. – A parenchymal band is a linear opacity, usually 1–3 mm thick and up to 5 cm long that usually extends to the visceral pleura (which is often thickened and may be retracted at the site of contact) (arrows, Fig. 48). It reflects pleuroparenchymal fibrosis and is usually associated with distortion of the lung architecture. Parenchymal bands are most frequently encountered in individuals who have been exposed to asbestos.122. and 123.
 |
| Fig. 48 |
PARENCHYMAL OPACIFICATION
Radiographs and CT. – Parenchymal opacification of the lungs may or may not obscure the margins of vessels and airway walls. 69Consolidation indicates that definition of these margins (excepting air bronchograms) is lost within the dense opacification, whereas ground-glass opacity indicates a smaller increase in attenuation, in which the definition of underlying structures is preserved. 83 The more specific terms consolidation and ground-glass opacity are preferred. (See also consolidation,ground-glass opacity.)
PERIBRONCHOVASCULAR INTERSTITIUM
Anatomy. – The peribronchovascular interstitium is a connective-tissue sheath that encloses the bronchi, pulmonary arteries, and lymphatic vessels. It extends from the hila to the lung periphery.
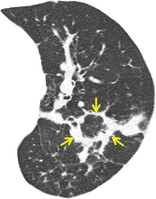
PERILOBULAR DISTRIBUTION
Anatomy. – The perilobular region comprises the structures bordering the periphery of the secondary pulmonary lobule.
CT. – This pattern is characterized by distribution along the structures that border the pulmonary lobules (arrows) (i.e., interlobular septa, visceral pleura, and vessels). 124 The term is most frequently used in the context (e.g., perilobular organizing pneumonia) that are distributed mainly around the inner surface of the secondary pulmonary lobule125 (Fig. 49). This may resemble indistinct thickening of the interlobular septa.
 |
| Fig. 49 |
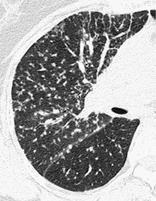
PERILYMPHATIC DISTRIBUTION
Anatomy. – This pattern is characterized by distribution along or adjacent to the lymphatic vessels in the lung. The routes of lymphatics are found along bronchovascular bundles, in the interlobular septa, around larger pulmonary veins, and in the pleura; alveoli do not have lymphatics.
CT. – Abnormalities along the pathway of the pulmonary lymphatics – that is, in the perihilar, peribronchovascular, and centrilobular interstitium, as well as in the interlobular septa and subpleural locations – have a perilymphatic distribution. 2 A perilymphatic distribution is typically seen in sarcoidosis (Fig. 50) and lymphangitic spread of cancer.
 |
| Fig. 50 |
PLEURAL PLAQUE
Pathology. – A pleural plaque is a fibrohyaline, relatively acellular lesion arising predominantly on the parietal pleural surface, particularly on the diaphragm and underneath ribs. 126 Pleural plaques are almost invariably the consequence of previous (at least 15 years earlier) asbestos exposure.
Radiographs and CT. – Pleural plaques are well-demarcated areas of pleural thickening, seen as elevated flat or nodular lesions that often contain calcification (Fig. 51). Plaques are of variable thickness, range from less than 1 to approximately 5 cm in diameter (arrow), and are more easily identified on CT scans than on chest radiographs. 127 An en face plaque may simulate a pulmonary nodule on chest radiographs. (See also pseudoplaque.)
 |
| Fig. 51 |
PNEUMATOCELE
Pathology. – A pneumatocele is a thin-walled, gas-filled space in the lung. It is most frequently caused by acute pneumonia, trauma, or aspiration of hydrocarbon fluid and is usually transient. The mechanism is believed to be a combination of parenchymal necrosis and check-valve airway obstruction. 128
Radiographs and CT. – A pneumatocele appears as an approximately round, thin-walled airspace (arrows) in the lung (Fig. 52).
 |
| Fig. 52 |
PNEUMOMEDIASTINUM
Pathology. – Pneumomediastinum is the presence of gas in mediastinal tissue outside the esophagus and tracheobronchial tree. It may be caused by spontaneous alveolar rupture, with subsequent tracking of air along the bronchovascular interstitium to the mediastinum. Pneumomediastinum is particularly associated with a history of asthma, severe coughing, or assisted ventilation.
Radiographs and CT. – Pneumomediastinum appears as lucent streaks on chest radiographs, mostly vertically oriented (Fig. 53). Some of these streaks may outline vessels and main bronchi. (See also pneumopericardium.)
 |
| Fig. 53 |
PNEUMONIA
Pathology. – Pneumonia is inflammation of the airspaces and/or interstitium (e.g., due to infection, as in bacterial pneumonia). Infective pneumonia is characterized by exudates resulting in consolidation. The term is also used to refer to a number of noninfectious disorders of the lung parenchyma characterized by varying degrees of inflammation and fibrosis (e.g., idiopathic interstitial pneumonias). 33
PNEUMOPERICARDIUM
Pathology. – Pneumopericardium is the presence of gas in the pericardial space. It usually has an iatrogenic, often surgical, origin in adults.
Radiographs and CT. – Pneumopericardium is usually distinguishable from pneumomediastinum because the lucency (low attenuation) caused by air does not extend outside the pericardial sac (Fig. 54). (See also pneumomediastinum.)
 |
| Fig. 54 |
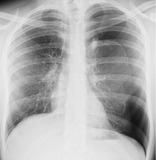
PNEUMOTHORAX AND TENSION PNEUMOTHORAX
Pathophysiology. – Pneumothorax refers to the presence of gas in the pleural space. Qualifiers include spontaneous, traumatic, diagnostic, and tension. Tension pneumothorax is the accumulation of intrapleural gas under pressure. In this situation, the ipsilateral lung will, if normal, collapse completely; however, a less than normally compliant lung may remain partially inflated.
Radiographs and CT. – On chest radiographs, a visceral pleural edge is visible (Fig. 55) unless the pneumothorax is very small or the pleural edge is not tangential to the X-ray beam. Tension pneumothorax may be associated with considerable shift of the mediastinum and/or depression of the hemidiaphragm. Some shift can occur without tension because the pleural pressure in the presence of pneumothorax becomes atmospheric, while the pleural pressure in the contralateral hemithorax remains negative.
 |
| Fig. 55 |
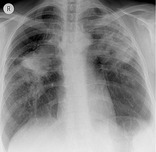
PROGRESSIVE MASSIVE FIBROSIS
Pathology. – This condition is caused by slow-growing conglomeration of dust particles and collagen deposition in individuals (mostly coal workers) heavily exposed to inorganic dust. 129
Radiographs and CT. – Progressive massive fibrosis manifests as masslike lesions, usually bilateral and in the upper lobes (Fig. 56). Background nodular opacities reflect accompanying pneumoconiosis, with or without emphysematous destruction adjacent to the massive fibrosis. 130 Lesions similar to progressive massive fibrosis sometimes occur in other conditions, such as sarcoidosis and talcosis.130. and 131.
 |
| Fig. 56 |
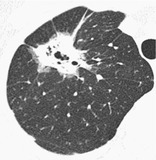
PSEUDOCAVITY
CT. – A pseudocavity appears as an oval or round area of low attenuation in lung nodules, masses, or areas of consolidation that represent spared parenchyma, normal or ectatic bronchi, or focal emphysema rather than cavitation. These pseudocavities usually measure less than 1 cm in diameter. They have been described in patients with adenocarcinoma (Fig. 57), bronchioloalveolar carcinoma, 132 and benign conditions such as infectious pneumonia.
 |
| Fig. 57 |
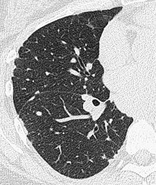
PSEUDOPLAQUE
CT. – A pseudoplaque is a pulmonary opacity contiguous with the visceral pleura formed by coalescent small nodules. It simulates the appearance of a pleural plaque. This entity is encountered most commonly in sarcoidosis (Fig. 58), silicosis, and coal-worker’s pneumoconiosis. 109
 |
| Fig. 58 |
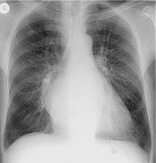
PULMONARY BLOOD FLOW REDISTRIBUTION
Pathophysiology. – Pulmonary blood flow redistribution refers to any departure from the normal distribution of blood flow in the lungs that is caused by an increase in pulmonary vascular resistance elsewhere in the pulmonary vascular bed.
Radiographs and CT. – Pulmonary blood flow redistribution is indicated by a decrease in the size and/or number of visible pulmonary vessels in one or more lung regions (Fig. 59), with a corresponding increase in number and/or size of pulmonary vessels in other parts of the lung. Upper lobe blood diversion in patients with mitral valve disease is the archetypal example of redistribution.133. and 134.
 |
| Fig. 59 |
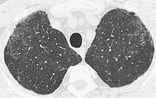
RESPIRATORY BRONCHIOLITIS–INTERSTITIAL LUNG DISEASE, OR RB-ILD
Pathology. – RB-ILD is a smoking-related disease characterized by inflammation (predominantly by macrophages) of the respiratory bronchioles and peribronchiolar alveoli, 33 sometimes with elements of or overlap with nonspecific and desquamative interstitial pneumonias. 135
CT. – RB-ILD typically manifests as extensive centrilobular micronodules and patchy ground-glass opacity corresponding to macrophage-rich alveolitis (Fig. 60), with or without fine fibrosis.136. and 137. It is often accompanied by bronchial wall thickening and minor centrilobular emphysema. Areas of air-trapping reflect a bronchiolitic component.
 |
| Fig. 60 |
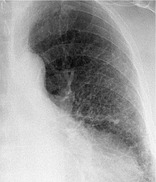
RETICULAR PATTERN
Radiographs and CT. – On chest radiographs, a reticular pattern is a collection of innumerable small linear opacities that, by summation, produce an appearance resembling a net (synonym: reticulation) (Fig. 61). This finding usually represents interstitial lung disease. The constituents of a reticular pattern are more clearly seen at thin-section CT, whether they are interlobular septal thickening, intralobuar lines, or the cyst walls of honeycombing. (Reticulation pattern and honeycombing should be considered synonymous. See also honeycombing.)
 |
| Fig. 61 |
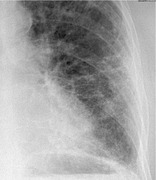
RETICULONODULAR PATTERN
Radiographs and CT. – A combined reticular and nodular pattern, the reticulonodular pattern is usually the result of the summation of points of intersection of innumerable lines, creating the effect on chest radiographs of superimposed micronodules (Fig. 62). The dimension of the nodules depends on the size and number of linear or curvilinear elements. (See also reticular pattern.) On CT scans, the pattern appears as a concurrence of reticular and micronodular patterns. The micronodules can be situated in the center of the reticular elements (e.g., centrilobular micronodules) or superimposed on the linear opacities (e.g., septal micronodules).
 |
| Fig. 62 |
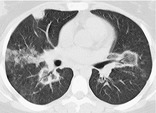
REVERSED HALO SIGN
CT. – The reversed halo sign is a focal rounded area of ground-glass opacity surrounded by a more or less complete ring of consolidation (Fig. 63). A rare sign, it was initially reported to be specific for cryptogenic organizing pneumonia138. and 139. but was subsequently described in patients with paracoccidioidomycosis. 140 Similar to the halo sign, this sign will probably lose its specificity as it is recognized in other conditions. (See also halo sign.)
 |
| Fig. 63 |
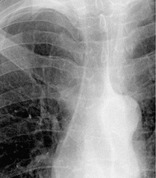
RIGHT PARATRACHEAL STRIPE
Anatomy and radiographs. – The right paratracheal stripe is a vertical, linear, soft tissue opacity less then 4 mm wide. It corresponds to the right tracheal wall, contiguous mediastinal tissues, and adjacent pleura (Fig. 64). The stripe is 3–4 cm in height and extends from approximately the level of the medial ends of the clavicles to the right tracheobronchial angle on a frontal chest radiograph. 141 It is seen in up to 94% of adults but is widened or absent in individuals with abundant mediastinal fat. The commonest pathologic cause of widening, deformity, or obliteration of this stripe is paratracheal lymph node enlargement.
 |
| Fig. 64 |
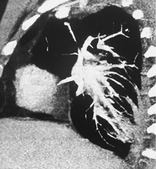
ROUNDED ATELECTASIS
Pathology. – Rounded atelectasis is rounded collapsed lung associated with invaginated fibrotic pleura and thickened and fibrotic interlobular septa. Most frequently, it is the consequence of an asbestos-induced exudative pleural effusion with resultant pleural scarring, 142 but it may occur with any cause of pleural fibrosis.
Radiographs and CT. – On chest radiographs, rounded atelectasis appears as a mass abutting a pleural surface, usually in the posterior part of a lower lobe. Distorted vessels have a curvilinear disposition as they converge on the mass (the comet tail sign). The degree of lobar retraction depends on the volume of atelectatic lung. It is almost invariably associated with other signs of pleural fibrosis (e.g., blunting of costophrenic angle). CT is more sensitive for the detection and display of the characteristic features of rounded atelectasis143. and 144. (Fig. 65). An additional sign is homogeneous uptake of contrast medium in the atelectatic lung. Synonyms include folded lung syndrome, helical atelectasis, Blesovsky syndrome, pleural pseudotumor, and pleuroma.
 |
| Fig. 65 |
SEGMENT
Anatomy. – A segment is the unit of a lobe ventilated by a segmental bronchus, perfused by a segmental pulmonary artery, and drained by an intersegmental pulmonary vein. There are two to five segments per lobe.
Radiographs and CT. – Individual segments cannot be precisely delineated on chest radiographs and CT scans, and their identification is made inferentially on the basis of the position of the supplying segmental bronchus and artery. When occasionally present, intersegmental fissures help to identify segments.
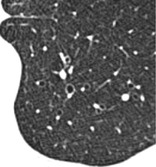
SIGNET RING SIGN
CT. – This finding is composed of a ring-shaped opacity representing a dilated bronchus in cross-section and a smaller adjacent opacity representing its pulmonary artery, with the combination resembling a signet (or pearl) ring145 (Fig. 66). It is the basic CT sign of bronchiectasis.27. and 146. The signet ring sign can also be seen in diseases characterized by abnormal reduced pulmonary arterial flow (e.g., proximal interruption of pulmonary artery147 or chronic thromboembolism). 148 Occasionally, the tiny vascular opacity abutting a bronchus is a bronchial, rather than a pulmonary, artery.
 |
| Fig. 66 |
SILHOUETTE SIGN
Radiographs. – The silhouette sign is the absence of depiction of an anatomic soft tissue border. It is caused by consolidation and/or atelectasis of the adjacent lung (Fig. 67), by a large mass, or by contiguous pleural fluid.149. and 150. The silhouette sign results from the juxtaposition of structures of similar radiographic attenuation. The sign actually refers to the absence of a silhouette. It is not always indicative of disease (e.g., unexplained absence of right border of heart is seen in association with pectus excavatum and sometimes in healthy individuals).
 |
| Fig. 67 |
SMALL-AIRWAYS DISEASE
Pathology. – This phrase is an arbitrary term used more frequently in thin-section CT descriptions than in the pathophysiology literature, where it was first coined. 151 Small-airways disease now generally refers to any condition affecting the bronchioles, whereas bronchiolitis more specifically describes inflammation of the bronchioles. 10
CT. – Small airways are considered to be those with an internal diameter smaller than or equal to 2 mm and a normal wall thickness of less then 0.5 mm. 32 Small-airways disease is manifest on CT scan as one or more of the following patterns: mosaic attenuation, air-trapping, centrilobular micronodules, tree-in-bud pattern, or bronchiolectasis.
SUBPLEURAL CURVILINEAR LINE
CT. – This finding is a thin curvilinear opacity, 1–3 mm in thickness, lying less than 1 cm from and parallel to the pleural surface (Fig. 68). It corresponds to atelectasis of normal lung if seen in the dependent posteroinferior portion of lung of a patient in the supine position and is subsequently shown to disappear on CT sections acquired with the patient prone. It may also be encountered in patients with pulmonary edema152 or fibrosis (other signs are usually present). Though described in the context of asbestosis, this finding is not specific for asbestosis.
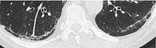 |
| Fig. 68 |
TRACTION BRONCHIECTASIS AND TRACTION BRONCHIOLECTASIS
CT. – Traction bronchiectasis and traction bronchiolectasis respectively represent irregular bronchial and bronchiolar dilatation caused by surrounding retractile pulmonary fibrosis. 153 Dilated airways are usually identifiable as such (arrows, Fig. 69) but may be seen as cysts (bronchi) or microcysts (bronchioles in the lung periphery). The juxtaposition of numerous cystic airways may make the distinction from ‘pure’ fibrotic honeycombing difficult.
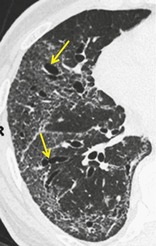 |
| Fig. 69 |
TREE-IN-BUD PATTERN
CT. – The tree-in-bud pattern represents centrilobular branching structures that resemble a budding tree. The pattern reflects a spectrum of endo- and peribronchiolar disorders, including mucoid impaction, inflammation, and/or fibrosis154. and 155. (Fig. 70). This pattern is most pronounced in the lung periphery and is usually associated with abnormalities of the larger airways. It is particularly common in diffuse panbronchiolitis, 156 endobronchial spread of mycobacterial infection, 157 and cystic fibrosis. A similar pattern is a rare manifestation of arteriolar (microangiopathic) disease. 158
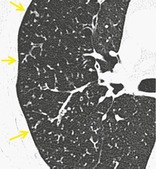 |
| Fig. 70 |
USUAL INTERSTITIAL PNEUMONIA, OR UIP
Pathology. – UIP is a histologic pattern of pulmonary fibrosis characterized by temporal and spatial heterogeneity, with established fibrosis and honeycombing interspersed among normal lung. Fibroblastic foci with fibrotic destruction of lung architecture, often with honeycombing, are the key findings. 33 The fibrosis is initially concentrated in the lung periphery. UIP is the pattern seen in idiopathic pulmonary fibrosis, but can be encountered in diseases of known cause (e.g., some cases of chronic hypersensitivity pneumonitis).
Radiographs and CT. – Honeycombing with a basal and subpleural distribution (Fig. 71) is regarded as pathognomonic,87. and 89. but not all cases of biopsy-proved UIP have this distinctive CT pattern.
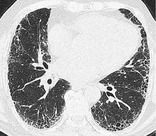 |
| Fig. 71 |
ACKNOWLEDGMENTS
The authors thank Peter Armstrong, MD, David A. Lynch, MD, Tom V. Colby, MD, and Tomas Franquet, MD, for their early critical review and members of the Fleischner Society, particularly Eric Milne, MD, and Paul Friedman, MD, for their helpful comments. Hannah Rouse, MD, assisted with the collection of illustrations. Anthony V. Proto, MD, placed arrows on the illustrations.
HRCT PATTERNS OF DIFFUSE LUNG DISEASE
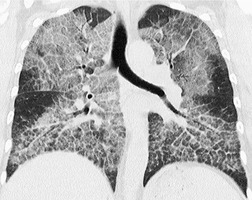
Diffuse abnormalities of the lung parenchyma on HRCT can be broadly categorized into one of the following four patterns: reticular and short linear opacities; nodular opacities; increased lung opacity (ground-glass opacity or consolidation); and cystic airspaces and areas of decreased lung density. Although these HRCT patterns mostly correspond to recognizable patterns on chest radiography, they are seen with much greater clarity on the cross-sectional images of HRCT and the precise distribution of disease is more readily appreciated.
Reticular pattern
A reticular pattern on HRCT almost invariably represents interstitial disease. The term is purely descriptive (reticulum = network) and there are several morphologic variations to this basic pattern, ranging from generalized thickening of the interlobular septa to honeycomb lung destruction (Box 4.1).
Box 4.1
• Thickened interlobular or intralobular septa
• Perilobular thickening
• Conspicuous ‘remnant’ septa (as in paraseptal or centrilobular emphysema)
• Miscellaneous causes of intersecting linear opacities and advanced cystic lung disease
• Honeycomb (fibrotic) destruction
A reticular pattern caused by thickening of interlobular septa is a frequent finding in many interstitial lung diseases. 94 Numerous thickened interlobular septa (which form polygonal outlines) indicate an extensive interstitial abnormality. Causes of interlobular septal thickening include infiltration with fibrosis, abnormal cells, and fluid (for example, interstitial fibrosis, lymphangitis carcinomatosa and pulmonary edema, respectively).
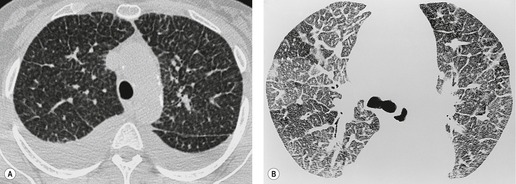
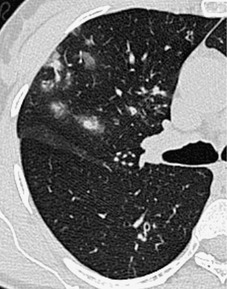
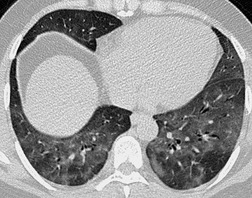
Thickened interlobular septa may appear smooth or irregular on HRCT94. and 159. but this distinction is not always obvious: irregular septal thickening is a feature of lymphangitic spread of cancer (Fig. 4.4)21.52.160. and 161. whereas pulmonary edema162 (Fig. 4.5) and alveolar proteinosis (Fig. 4.6) cause smoother septal thickening.72.163. and 164. Sarcoidosis can cause nodular septal thickening (Fig. 4.7) although thickened septa may not be particularly profuse in this disease. 61 Of the common conditions that cause generalized thickening of the interlobular septa, lymphangitis carcinomatosa is the prototype. Conditions characterized by thickened interlobular septa are shown in Box 4.2.
Box 4.2
Dominant feature
• Hydrostatic pulmonary edema165
• Venoocclusive disease166
• Pulmonary vein atresia167
• Lipoid pneumonia74
• Leukemia or lymphoma infiltration168
• Septal amyloidosis169
• Diffuse pulmonary lymphangiomatosis170
• Kaposi sarcoma171
• Congenital lymphangiectasia172
• Acute eosinophilic pneumonia173
• Congenital surfactant deficiencies176
Occasional feature
As a consequence of the continuity of the various parts of the lung interstitium, 17 widespread interstitial disease, which causes thickening of the interlobular septa, also results in bronchovascular interstitial thickening (typified by lymphangitis carcinomatosa) (Figs 4.4 and 4.8). This HRCT finding may be obvious, particularly in regional or unilateral interstitial lung disease but is sometimes quite subtle when it is either minimal or diffuse. The HRCT finding of bronchovascular thickening in isolation needs to be interpreted with caution since it may be a manifestation of reversible inflammatory airways disease, for example a lower respiratory tract infection or asthma. Thickening of the subsegmental and segmental bronchovascular bundles, such as that caused by lymphangitis carcinomatosa, often gives the interface between the bronchial wall and surrounding lung an irregular and ‘feathery’ appearance.
At the level of the pulmonary lobule, axial interstitial thickening of the centrilobular artery and bronchiole is seen as a prominent dot or Y-shaped opacity; there is often associated thickening of the interlobular septa. Such thickening is seen in a variety of interstitial lung diseases, and is recognized as one of the earliest manifestations of the fibrosis of asbestosis.123. and 194. Inflammatory diseases involving the smallest airways can produce apparent thickening of the core structures on HRCT; in these instances, the abnormal core structure visible on HRCT is due to thickening and dilatation of the central bronchiole, which is filled with, and surrounded by, exudate.156. and 197. Distinguishing between axial interstitial disease and exudative small airways disease, both of which give rise to prominent core structures, is rarely a problem: in the latter situation the larger subsegmental and segmental bronchi are usually abnormal.
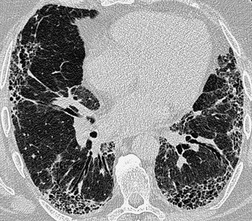
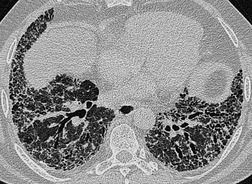
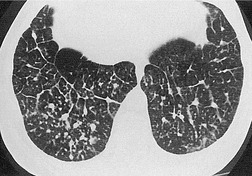
The size of network making up the reticular pattern on HRCT is determined by the level at which the interstitial thickening is most pronounced. Thickening of the intralobular septa will result in a fine reticular pattern on HRCT; such a pattern may occur in any interstitial disease but is typified by idiopathic pulmonary fibrosis. 192 Some of the very finest linear structures that make up this netlike pattern within the secondary pulmonary lobule will be so small as to be below the resolution limits of HRCT, even with the narrowest collimation. The result is an amorphous increase in lung density (ground-glass opacity) due to volume averaging within the section. 69 Interestingly, obvious thickening of the interlobular septa is not, contrary to expectation, a prominent feature in idiopathic pulmonary fibrosis (usual interstitial pneumonia), possibly because of the severe distortion of the lung architecture (Figs 4.9 and 4.10).
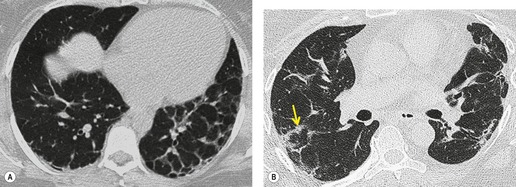
Extensive and severe pulmonary fibrosis, which causes destruction of the architecture of the pulmonary lobules, results in a characteristic coarse reticular pattern made up of irregular linear opacities. The reticular pattern of end-stage fibrotic (honeycomb) lung mirrors the appearances on chest radiography and is characterized by cystic spaces, measuring a few millimeters to several centimeters across, surrounded by thick irregular walls87.192. and 198. (Fig. 4.10). The distortion of normal lung morphology by extensive fibrosis may result in irregular dilatation of the segmental and subsegmental bronchi; in the lung periphery the dilated bronchioles may be difficult to distinguish from the surrounding parenchymal cystic airspaces199 (Fig. 4.11). The term ‘traction bronchiectasis’200 has been given to airways that are clearly dilated because of surrounding retractile pulmonary fibrosis. This phenomenon is not confined to the situation in which fibrosis is seen as a reticular or honeycomb pattern on HRCT; dilatation and distortion of airways within ground-glass opacification (often with a faint granular texture) is an important sign of fine intralobular fibrosis.
The positive and definite identification of a honeycombing, as opposed to other forms of reticulation, is of particular relevance in patients with idiopathic pulmonary fibrosis. 87 The positive predictive value of a subpleural basal honeycomb pattern on HRCT for the diagnosis of idiopathic pulmonary fibrosis (usual interstitial pneumonia at a histopathologic level) is high. 201 Whereas a subpleural nonhoneycomb reticular pattern may be encountered in other diseases, for example nonspecific interstitial pneumonia, 118 rarely in sarcoidosis177 or exceptionally in Wegener granulomatosis. 202 Because, by definition, a honeycomb pattern is made up of cystic airspaces, an attempt should be made to confirm air density within the cysts (by comparison with air within the large bronchi), before assigning the term honeycomb (Fig. 4.10).
In some diseases a perilobular distribution124 may give the spurious impression of thickening of the interlobular septa. The disposition of the pathologic process, such that it is ‘smeared’ around the internal lobular surface, creates the appearance of a thickened lobular margin (Fig. 4.12). The perilobular sign is encountered in cases of organizing pneumonia125 (Fig. 4.13). Although it was originally described in patients with, among other diseases, usual interstitial pneumonia, 124 it is not a common sign apart from in patients with organizing pneumonia (in whom a perilobular pattern is present, albeit usually limited to a few arcades, in approximately half of cases). 125 The crazy-paving pattern (a reticular pattern superimposed on a background or ground-glass opacity) may occasionally represent a similar perilobular distribution rather than actually thickened interlobular septa. 203 Another situation in which there may be apparent, rather than real, interlobular septal thickening occurs in patients with emphysema; the residual peripheral alveoli collapse against the lobule margins, and in so doing spuriously thicken the interlobular septa. Furthermore, in cigarette smokers there may also be accompanying interstitial fibrosis, 137 which is partly responsible for conspicuous thickening of the ‘remnant’ interlobular septa204 (Fig. 4.14).
An unusual HRCT finding is a fine peripheral curvilinear line lying within 1 cm from the visceral pleural surface. This dense line is extremely well defined and parallels the chest wall (Fig. 4.15). The subpleural curvilinear line caused confusion when it was first described in patients with asbestosis205 because it was suggested that such a line was virtually pathognomonic of asbestos-induced interstitial fibrosis. However, it is now recognized that this HRCT sign is seen in patients with interstitial disease of various causes122 and some normal individuals, 206 and it should not be regarded as specific for asbestosis.
Nodular pattern
A nodular pattern is a feature of both interstitial and airspace diseases. The localization of nodules as well as other characteristics such as their density, clarity of outline, and range or uniformity of size may indicate whether the nodules are lying predominantly within the interstitium or air spaces. 207 Since many diffuse lung diseases have both interstitial and airspace components, this distinction is not always helpful in refining a differential diagnosis. Nevertheless, a systematic approach to the distribution (perilymphatic versus centrilobular) and other characteristics of multinodular disease is recommended. 208 Whether pulmonary nodules can be detected on CT depends on their size, profusion, density, and CT technique. Narrow collimation is clearly superior to thick section acquisition for the detection of very small nodules because there is less partial volume effect which can average out the attenuation of tiny nodules. 209 A further refinement is the use of maximum intensity projection (MaxIP) reconstructions of contiguous thin sections acquired by volumetric CT; these images improve the depiction of minute nodules but tend not to be routinely performed210. and 211. (Fig. 4.16).
Nodules within the lung interstitium, especially those related to the lymphatic vessels, are seen in the interlobular septa, subpleural regions, and in a peribronchovascular distribution. Nodular thickening of the bronchovascular interstitium results in an irregular interface between the margins of the bronchovascular bundles and the surrounding lung parenchyma. This irregularity, which has been named the ‘interface sign’, 212 may be seen in lymphangitis carcinomatosa,21. and 52. but is most obvious in cases of sarcoidosis when a coalescence of perilymphatic granulomas results in a beaded appearance of the thickened bronchovascular bundles61. and 213. (Fig. 4.17). The bronchovascular distribution of nodules in conjunction with subpleural nodules (most obvious along the oblique fissures) and a perihilar concentration of disease are characteristic of sarcoidosis. The nodular pattern seen in coal worker’s pneumoconiosis and silicosis is generally more uniform in distribution; the nodules may be more upper zone and subpleural in distribution, but overall tend to be more randomly spread throughout the lung parenchyma than those seen in sarcoidosis. Thickening of the interlobular septa can be quite pronounced in silicosis109. and 179. (Fig. 4.18).
A random distribution of small, well-defined (miliary) nodules is seen in patients with hematogenous spread of tuberculosis,214. and 215. some forms of pulmonary metastases, pneumoconiosis, and rarely in pulmonary sarcoidosis (in which case there is usually some thickening of the septa and fissures). Subtle features of thickening of the interlobular and intralobular interstitium may be associated with a miliary or micronodular pattern. 216 The size and cephalocaudal distribution of miliary nodules are generally not useful characteristics for narrowing the differential diagnosis. 217 Micronodules of calcific density are a feature of alveolar microlithiasis178 but unless they coalesce their high attenuation may not be evident. The curious appearance of dotlike and branching dense opacities, not related to any part of lobular anatomy, and usually on a background of pulmonary fibrosis, is pathognomonic of dendriform pulmonary ossification. 218
When the airspaces are filled, or partially filled, with the products of disease, individual acini may become visible on HRCT as poorly defined nodules approximately 8 mm in diameter (Fig. 4.19). Acinar nodules may merge with areas of ground-glass opacification and are often seen around the periphery of areas of dense parenchymal consolidation (e.g. in reactivation pulmonary tuberculosis). Smaller poorly defined, often centrilobular, nodules are encountered in many conditions;208.219.220. and 221. their centrilobular location may not be obvious when the nodules are profuse but sparing of the immediate subpleural lung suggests that the nodules are predominantly centrilobular. Conditions in which centrilobular nodules of varying quality and size221 (ranging from the indistinct low-density nodules seen in subacute hypersensitivity pneumonitis [Fig. 4.20], through to the well-defined Y-shaped opacities of diffuse panbronchiolitis222 [Fig. 4.3]) are listed in Table 4.2).
| Diffuse panbronchiolitis | Peripheral Y-shaped opacities, accompanied by156. and 197. features of widespread cylindrical bronchiectasis |
| Subacute hypersensitivity pneumonitis223 and hot tub lung224 | Low-density, poorly defined nodules, usually superimposed on background of ground-glass opacity |
| Endobronchial spread of TB157. and 225. or, less frequently, bacterial pneumonia226. and 227. | Some nodules from confluent consolidation. In the case of tuberculosis, larger cavitating nodules usually present |
| Atypical mycobacterial infection228.229. and 230. | Associated with bronchial abnormalities, 1 cm diameter nodules and architectural distortion |
| Human T lymphotropic virus type 1 carriers187 | Centrilobular nodules with thickening of bronchovascular bundles, caused by lymphocytic infiltration |
| Acute Mycoplasma pneumoniae infection231 | Poorly defined centrilobular nodules and tree-in-bud pattern |
| Various viral pneumonias232 especially postbone marrow transplant173.186. and 233. | Centrilobular nodules, tree-in-bud pattern, ground-glass opacities and consolidation |
| Cryptogenic organizing pneumonia119 | An unusual pattern (similar to the nodules seen in subacute hypersensitivity pneumonitis) |
| Respiratory bronchiolitis–interstitial lung disease234. and 235. | Poorly defined, low-density nodules associated with bronchial wall thickening, thickened interlobular septa and mosaic pattern |
| Churg–Strauss syndrome181 and eosinophilic pneumonia236 | Indistinct nodules, often with other parenchymal and airways abnormalities |
| Bronchioloalveolar cell carcinoma237. and 238. | Usually in association with areas of ground-glass opacity or consolidation |
| Lymphoid interstitial pneumonia182 (including follicular bronchiolitis) 239 (in adults) 105 or in children with HIV240 | Profuse micronodules with perilymphatic and subpleural distribution |
| Acute silicoproteinosis241 | Poorly defined centrilobular nodules with accompanying ground-glass opacity and consolidation |
| Various small vessel diseases242.243. and 244. | Intravascular filling, e.g. with foreign material or tumor emboli may result in a tree-in-bud pattern |
| Low-grade aspiration245. and 246. | Tree-in-bud pattern and poorly defined nodules representing bronchiolar filling |
| Primary pulmonary lymphoma or leukemia247. and 248. | Tree-in-bud pattern simulating diffuse panbronchiolitis but without cylindrical bronchiectasis |
| Fat embolism249 | Small nodules, some related to vessels, admixed with ground-glass opacity and consolidation. |
Parenchymal opacification
Ground-glass opacity
A hazy increase in the density of the lung parenchyma on HRCT is described as a ‘ground-glass’ opacification. Unlike the analogous abnormality on chest radiography, in which the pulmonary vessels are often indistinct, ground-glass opacity on HRCT does not obscure the pulmonary vasculature83. and 250. (Fig. 4.21). In cases in which the presence of ground-glass opacity is equivocal, it may be helpful to compare the attenuation of the lung parenchyma with air in the bronchi: in normal individuals the difference in density is marginal, whereas ground-glass opacity makes the airways more obvious (the ‘black bronchus’ sign) (Fig. 4.21). Although this HRCT abnormality is usually easily recognizable, particularly when it is interspersed with areas of normal lung parenchyma (see next section on mosaic attenuation pattern), subtle degrees of increased parenchymal opacification may not be obvious251 (Fig. 4.22) and the conspicuity of ground-glass opacity is particularly susceptible to alterations in window settings. Furthermore, a normal increase in lung density, indistinguishable from ‘pathologic’ ground-glass opacification, is seen in individuals breathholding at near-residual volume251 (Fig. 4.23). There are several causes of spurious ground-glass opacification on HRCT including inappropriate (wide) window settings, contrast and brightness settings, drift on workstation monitors, and the appearance of the lung parenchyma on images obtained on an unfamiliar CT scanner.
At a microscopic level, the changes responsible for ground-glass opacity may be complex and include partial filling of the airspaces, considerable thickening of the interstitium, or a combination of the two69. and 83. (Fig. 4.24). Nevertheless, the basic mechanism behind the generation of the HRCT pattern of ground-glass opacification of the lungs is nothing more or less than the displacement of air. Thickening of the intralobular interstitium by fluid or a cellular infiltrate is below the limits of resolution of HRCT and volume averaging results in an amorphous increase in lung density. Many conditions that are characterized by these pathologic changes result in the nonspecific pattern of ground-glass opacity. 252 Conditions that are characterized by ground-glass opacity as the dominant abnormality on HRCT are shown in Box 4.3.
Box 4.3
Given the numerous conditions listed in Box 4.3, it is clearly a mistake to consider ground-glass opacity as simply representing ‘active alveolitis’, although this popular, if oversimplistic, interpretation has proved remarkably durable. Nevertheless, the pattern of ground-glass opacity may represent potentially reversible lung disease, 69 but there are important exceptions to this rule: widespread fine intralobular fibrosis may produce a ground-glass pattern (frequently the nonspecific interstitial pneumonitis subtype of the interstitial pneumonias), but in this situation there is distortion and dilatation of the bronchi84 (Fig. 4.25




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree