HIV/AIDS
Infection with the human immunodeficiency virus (HIV) that causes the acquired immunodeficiency syndrome (AIDS) has been, and continues to be, one of the great scourges of the late twentieth and early twenty-first centuries. HIV/AIDS is estimated to have caused over 20 million deaths through 2004, and almost 3 million deaths in 2006 alone. 1 The latter half of the 1990s saw remarkable advances in the treatment of HIV infection, with aggressive use of prophylactic antimicrobial therapies and the introduction of highly effective antiretroviral therapies (ART).1.2.3.4.5.6.7.8. and 9. Thus, over the past 10 years, the morbidity and mortality of HIV infection has fallen dramatically in populations with access to these treatments. 9 Unfortunately, the high mortality of the disease continues unabated in populations with limited access to care, such as sub-Saharan Africa.
The pulmonary manifestations of HIV/AIDS can now be considered to have two types of presentation. 9 In underserved populations, the manifestations are much as they were in the 1980s: opportunistic infections, particularly Pneumocystis jirovecii pneumonia, tuberculosis, disseminated fungal infection, and AIDS-related neoplasms such as Kaposi sarcoma (see Box 6.1). 10 In populations with access to ART, however, the pulmonary manifestations of HIV infection have changed dramatically (see Box 6.2). In these groups, opportunistic fungal infections have become much less common; bacterial infection is now the commonest cause of pulmonary infection and hospital admission in these patients. 1,9,11 Also, HIV-related neoplasms such as Kaposi sarcoma have declined in incidence, while other non-AIDS-defining malignancies such as lung cancer may be increasing in frequency. 1,9,12,13 New manifestations of the disease have also become evident in these groups, perhaps because of longer survival, including an increased incidence of emphysema and pulmonary hypertension.14.15. and 16. The immune reconstitution inflammatory syndrome (IRIS), which occurs during restoration of the immune system, can cause considerable morbidity in patients treated with ART.3.4.5.6.7.8. and 9.,17,18 Finally, ART itself is increasingly implicated as a cause of respiratory symptoms and disease, including nucleoside-induced lactic acidosis, an increased incidence of bacterial pneumonia, and hypersensitivity reactions. 9
Box 6.1
Infection
• Bacterial (pyogenic) pneumonia
– Streptococcus pneumoniae
– Haemophilus influenzae
– Pseudomonas aeruginosa
– Staphylococcus aureus
– Moraxella catarrhalis
– Rhodococcus equi
• Mycobacteria
– Mycobacterium tuberculosis
– Mycobacterium kansasii
– Mycobacterium avium complex (MAC)
– Other non-tuberculous mycobacteria
• Fungal infection
– P. jirovecii
– Cryptococcus neoformans
– Histoplasma capsulatum
– Aspergillus fumigatus
– Coccidioides immitis
– Blastomyces dermatitides
• Protozoal
– Strongyloides stercoralis
– Toxoplasma gondii
• Viral infection
– Cytomegalovirus (CMV)
– Adenovirus
– Herpes simplex virus
Malignancy
• Kaposi sarcoma
• Non-Hodgkin lymphoma, including primary effusion lymphoma
• Lung cancer
Other disorders
• Sinusitis
• Bronchitis
• Bronchiectasis
• Emphysema
• Lymphoid interstitial pneumonia
• Nonspecific interstitial pneumonia
• Cryptogenic organizing pneumonia
• Pulmonary hypertension
• Immune reconstitution inflammatory syndrome (IRIS)
*Adapted from Rosen.1
Box 6.2
Less common
• Opportunistic infection, especially P. jirovecii, tuberculosis
• Kaposi sarcoma
Similar incidence
• Non-Hodgkin lymphoma
More common
• Non-AIDS-defining cancers (e.g. lung cancer)
• Immune reconstitution inflammatory syndrome (IRIS)
• Pulmonary hypertension
• Emphysema
• ART-related respiratory disease
– Nucleoside-induced lactic acidosis
– Increased incidence of bacterial pneumonia (enfuvirtide)
– Hypersensitivity reactions (abacavir)
HIV is a retrovirus that attaches to the CD4+ surface glycoprotein of helper T lymphocytes, monocytes, macrophages, and other antigen presenting cells. It fuses with the cell, injects its RNA (ribonucleic acid), makes a DNA (deoxyribonucleic acid) copy, and integrates into the host DNA. The virus replicates within the helper T lymphocytes and eventually destroys the infected cells. It also causes ‘bystander’ killing of uninfected cells by autoimmune destruction and apoptosis resulting in the characteristic CD4+ lymphocyte depletion. Cell-mediated immunity becomes progressively impaired as the CD4+ lymphocyte count falls. The types of infection to which HIV-infected patients become susceptible vary as cell-mediated immunity becomes less effective at eradicating viruses, fungi, protozoa, and facultative intracellular bacteria such as Mycobacterium tuberculosis. Knowledge of the CD4+ lymphocyte count can thus be helpful for interpreting imaging in AIDS patients (see Box 6.3). 1,19.20. and 21. The normal CD4+ lymphocyte count is greater than 500 cells/µL. As the CD4+ lymphocyte count falls, tuberculous infection, bacterial pneumonia, and oral Candida albicans infection become increasingly common, as do virus-associated tumors such as Kaposi sarcoma. P. jirovecii (previously known as Pneumocystis carinii) is a common cause of pneumonia when the CD4+ lymphocyte count is 200 cells/µL or less (assuming no antibiotic prophylaxis). With counts below this level, HIV infection becomes frank AIDS and the CD8-cytotoxic lymphocyte count also falls. When the CD4+ count falls below 100 cells/µL, toxoplasmosis, disseminated herpes zoster, cryptococcosis, esophageal candidiasis, cytomegalovirus (CMV), atypical mycobacterial infections, and Epstein–Barr virus-related non-Hodgkin lymphoma become more frequent.
Box 6.3
CD4+ >500 cells/µL
• Sinusitis/pharyngitis
• Bronchitis
• Lung cancer
CD4+ <400 cells/µL
• Bacterial (pyogenic) pneumonia
• Pulmonary tuberculosis
• Cardiomyopathy
CD4+ <200 cells/µL
• P. jirovecii pneumonia
• Kaposi sarcoma
• Bacterial sepsis
• Disseminated tuberculosis
CD4+ <100 cells/µL
• Disseminated MAC
• CMV
• Disseminated fungal infection
• Non-Hodgkin lymphoma
*Adapted from Rosen.1
Bacterial (pyogenic) infection
Although T cell defects are the major cause of immune deficiency in patients with HIV infection, B cell function and antibody production are also affected, particularly in children. This deficiency in humoral immunity, in addition to the reduction in CD4+ lymphocyte counts, increases susceptibility to bacterial infection. Other factors that may predispose AIDS patients to pyogenic infection include poor drainage of lung due to endobronchial obstruction by Kaposi sarcoma and bone marrow suppression from drugs used to treat CMV infection and various tumors. In particular, neutropenia due to bone marrow suppression may predispose AIDS patients to Gram-negative bacterial pneumonia and sepsis.
In one large prospective North American series (pre-ART), pyogenic bacterial pneumonia was six times more common in HIV-positive than in HIV-negative patients. 22 Pyogenic organisms are responsible for up to 45% of pulmonary infections in AIDS patients at some stage of their illness with an even higher incidence when AIDS is contracted through intravenous drug abuse. 23,24 Although the overall incidence of pyogenic bacterial pneumonia in AIDS patients is declining, it remains an important cause of morbidity and mortality and, as noted above, is now the most common cause for hospital admission in HIV-infected patients receiving ART. 9,25,26
Many types of community-acquired bacterial infection are encountered in patients with HIV infection (see Box 6.1). 25 The most common pathogens are S. pneumonia and H. influenzae. 1,27,28Legionella pneumophila infection is uncommon, but frequently severe. 1,28,29P. aeruginosa is an important pathogen in severely immune suppressed patients who can develop disseminated infection. 1,25,30 Infection with other bacterial pathogens including R. equi and Nocardia is also reported. 1,31.32. and 33.
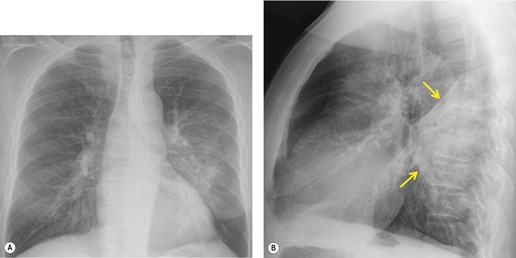
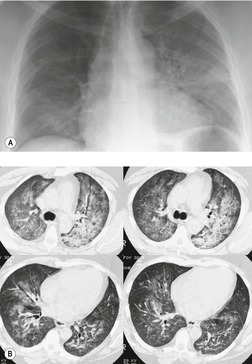
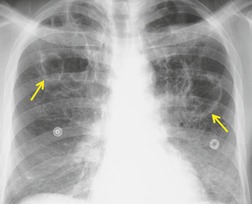
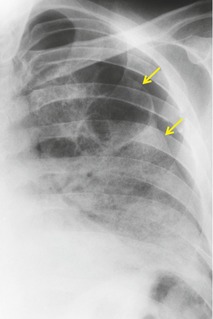
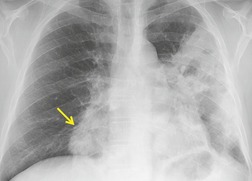
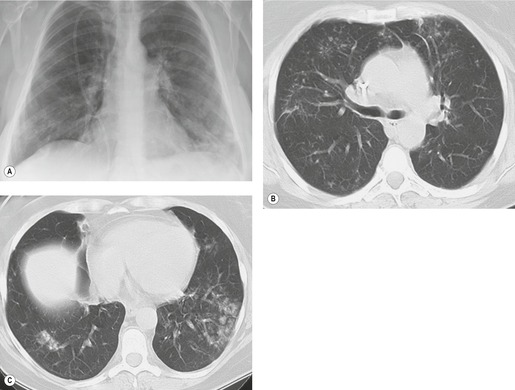
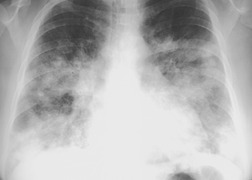
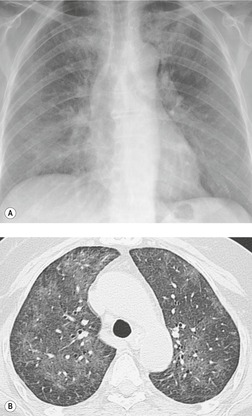
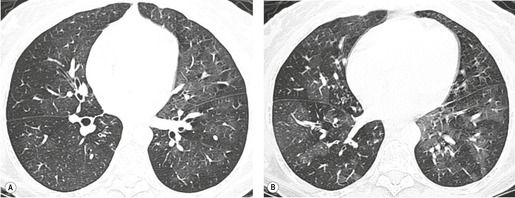
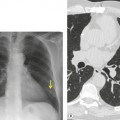
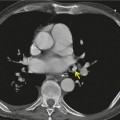
Chest radiography remains a mainstay for diagnosis of suspected community-acquired bacterial infection in HIV-infected persons. Computed tomography (CT) can on occasion reveal important findings not seen on chest radiographs, and can be useful for directing further diagnostic evaluation. 34 The imaging findings of bacterial pneumonia in HIV-infected patients are similar to those seen in immunocompetent patients and have not markedly changed in the ART era. 27 Focal homogeneous segmental or lobar consolidation is probably the most common pattern (Fig. 6.1). 23,27,35 However, other patterns including multifocal bronchopneumonia and either localized or diffuse heterogeneous opacities, sometimes indistinguishable from the findings of Pneumocystis pneumonia, are seen in up to half of patients, especially those infected with H. influenzae and P. aeruginosa. 24,27,28,36 Multifocal disease becomes more common as the CD4+ lymphocyte count falls. 27 Other less common imaging findings of bacterial infection include pleural effusion, lung nodules or masses, and pulmonary cavitation (Fig. 6.2). 23,24,36,37 Cavitation in patients with Pseudomonas pneumonia is less common in patients receiving ART. 30
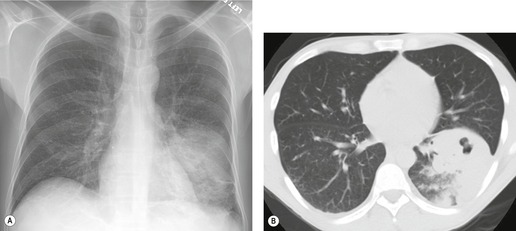
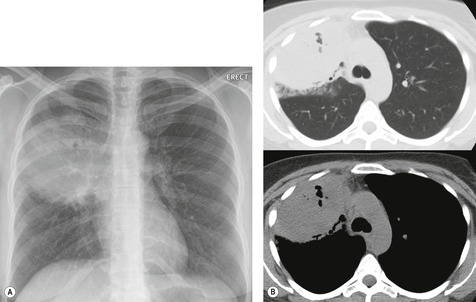
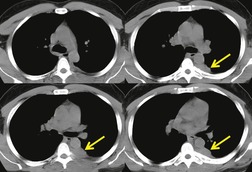
Hematogenous spread of bacterial pneumonia can cause septicemia, multifocal pulmonary infection, and systemic septic emboli to skin and solid organs. These complications are more common in patients with AIDS than in immunocompetent individuals. Sepsis may result in diffuse homogeneous pulmonary opacities on chest radiographs and lead to adult acute respiratory distress syndrome (ARDS). Septic pulmonary emboli are a particularly common complication in intravenous drug users with AIDS. Septic pulmonary emboli manifest on chest radiographs and CT as peripheral wedge-shaped opacities and cavitary lung nodules (Fig. 6.3). The cavities can be quite thin walled.
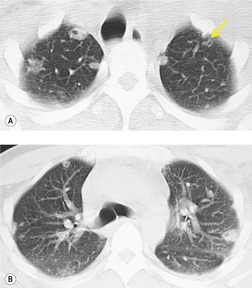
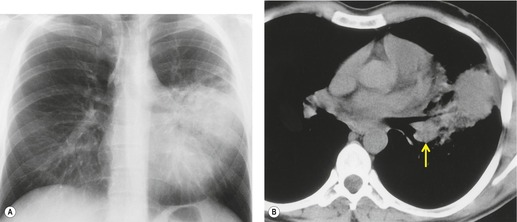
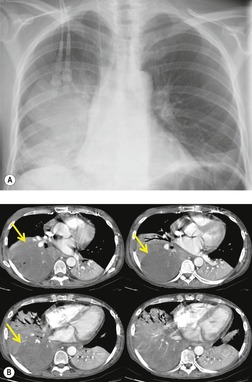
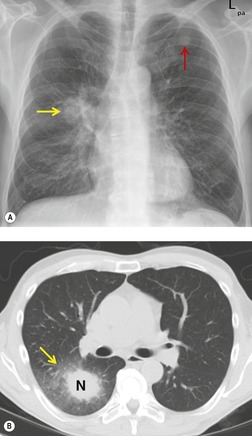
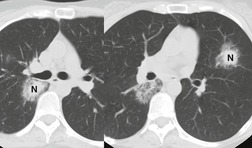
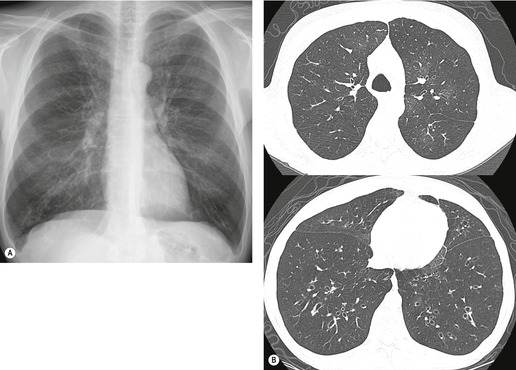
Nocardia asteroides typically causes clinically significant infection in AIDS patients with a very low CD4+ count (mean of 80 cells/µL). 1,38,39 The organisms can be identified on biopsy samples, sputum cultures, and bronchoalveolar lavage aspirates. Nocardia infection manifests on imaging as consolidation, large masses, or multiple nodules, all of which may cavitate (Fig. 6.4). 40,41 The upper lobes are more commonly affected than the lower lobes. Rapid progression of pulmonary abnormalities is typical, as are associated pleural effusions. Central nervous system dissemination can occur.
Bacillary angiomatosis is a vasoproliferative infection in patients with AIDS that is caused by Bartonella species.42.43. and 44. Exposure to cats, cat fleas, and lice are the main risk factors. The skin is the most common site of involvement. Bacillary angiomatosis in the thorax can manifest as mediastinal lymph node enlargement, lung nodules or masses, endobronchial lesions, chest wall masses, or pleural effusions. 45,46 Because of their vascularity, the mass lesions may enhance intensely on CT after administration of intravenous contrast material.
Mycobacterial infection (see Box 6.4)
Tuberculosis
In most developed countries, the incidence of tuberculosis steadily declined until 1985. After 1985, the incidence began to rise again, at least partly due to the advent of widespread HIV infection. In developed countries, the incidence again leveled off in the mid-1990s and began to slowly decline thereafter. The advent of ART has had a substantial effect on the incidence of tuberculosis in patients with access to therapy (see Box 6.2). 9 However, M. tuberculosis remains an important pathogen in the HIV/AIDS population in developed countries, and is still the major cause of death in AIDS patients in Africa (see Box 6.4). 47 Latent tuberculosis is an important cofactor in development of IRIS (see below). 17,18 The risk of tuberculous infection depends on the concentration of tubercle bacilli in the environment, which is heavily dependent on social conditions. The high rate of multidrug resistant tuberculosis in HIV-positive patients in cities such as New York is of great importance both to those with HIV infection and to the immunocompetent population. 48 Patients with AIDS-related tuberculosis49 are more likely to be nonwhite and also more likely to be heterosexual drug users than AIDS patients without tuberculosis. 50
Box 6.4
Tuberculosis
• Common cause of death – especially in sub-Saharan Africa and other underserved populations
• Much less common in patients receiving ART
• Can be manifestation of IRIS
• CD4+ lymphocyte count >200 cells/µL
– Postprimary pattern
– Upper lobe fibrocavitary disease
– Hematogenous dissemination rare
• CD4+ lymphocyte count <200 cells/µL
– Primary pattern
– Lymphadenopathy
– Hematogenous dissemination common (miliary)
– Chest radiograph normal in up to 10%
Nontuberculous mycobacteria
• MAC
– Commonest
– Disseminated disease when CD4+ <100 cells/µL
– Chest radiograph normal in 50%
– Lymphadenopathy most common manifestation
– Parenchymal lung disease uncommon
• M. kansasii
– Second commonest
– Similar to tuberculosis
The diagnosis of tuberculosis in HIV-infected patients can be difficult. 51,52 The various tuberculin tests, which rely on an intact cell-mediated response, are falsely negative in 50–70% of patients with advanced AIDS. Up to half of AIDS patients with culture proven tuberculosis have false-negative sputum and bronchoalveolar lavage samples for acid-fast bacilli. As many as 10% of patients with advanced AIDS and tuberculosis have normal chest radiographs. 53,54 Prompt diagnosis and treatment therefore requires a high index of clinical suspicion.
The clinical and imaging manifestations of tuberculosis in HIV-infected patients very much depend upon the level of immunosuppression (see Box 6.3).55.56.57.58.59.60.61.62. and 63. In individuals with only mild reductions in CD4+ lymphocyte counts, or in those receiving ART, disease is usually localized to the lung and is quite similar to tuberculosis in nonimmune-suppressed populations. 1,64.65. and 66. With more advanced reductions in cell-mediated immunity, however, disease is more likely to manifest with atypical patterns and be disseminated. 1,64,65 Up to 60% of AIDS patients with tuberculosis have at least one extrapulmonary site of disease, a much higher incidence than in patients who do not have AIDS. 50
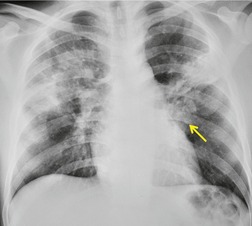
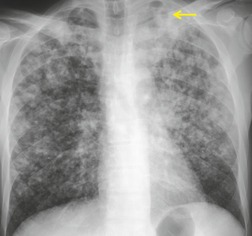
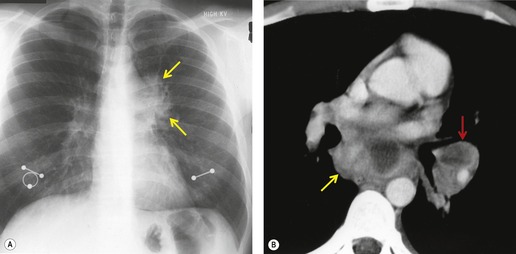
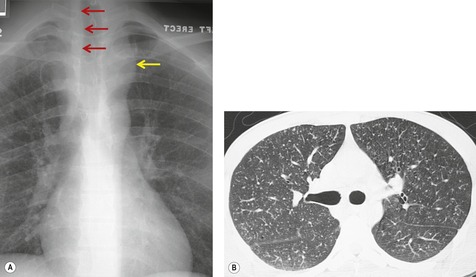
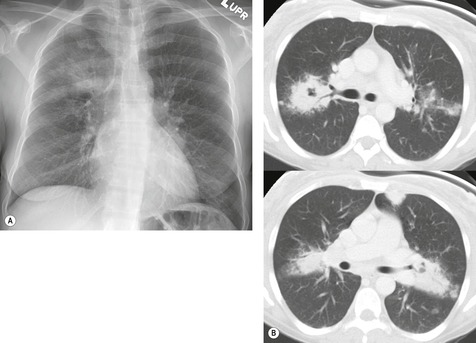
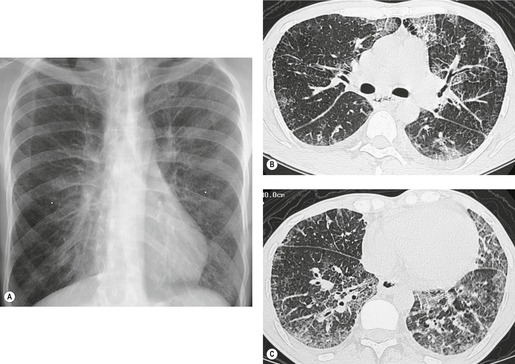
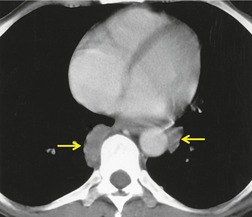
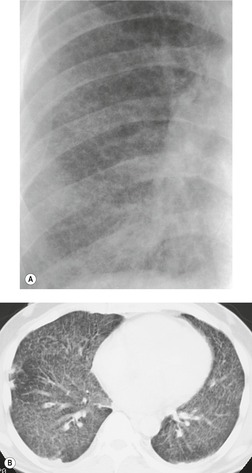
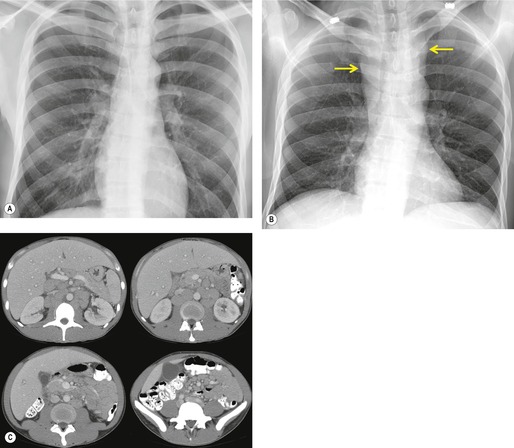
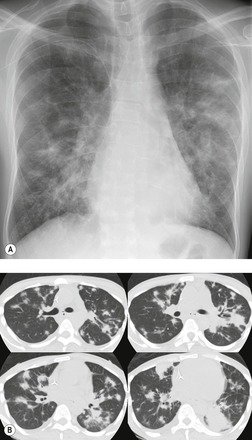
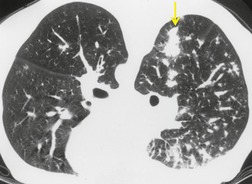
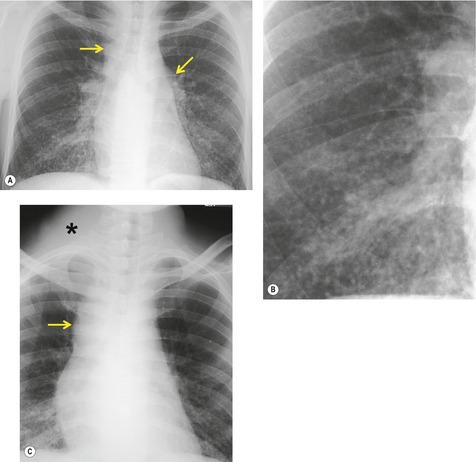
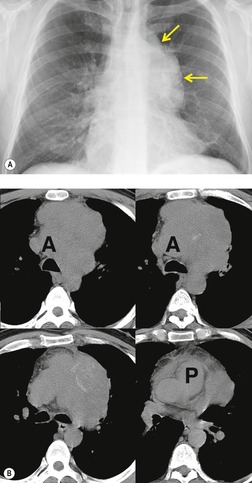
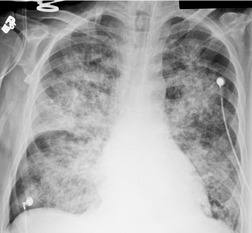
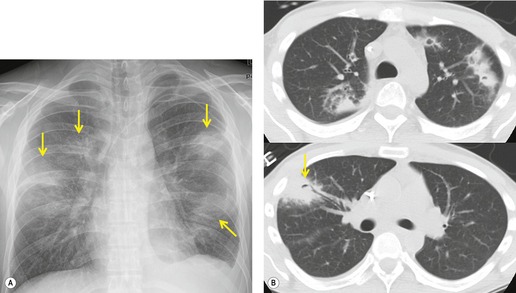
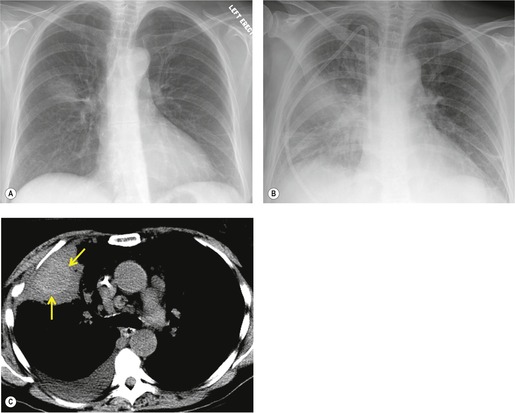
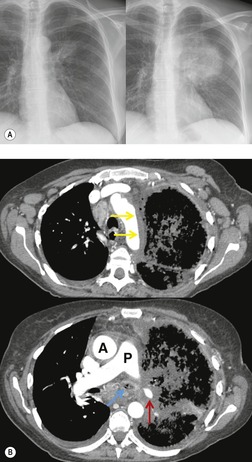
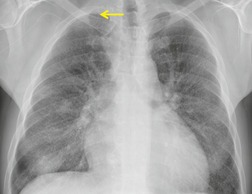
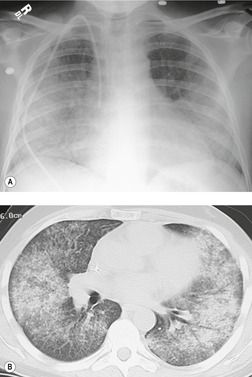
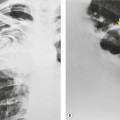
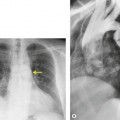
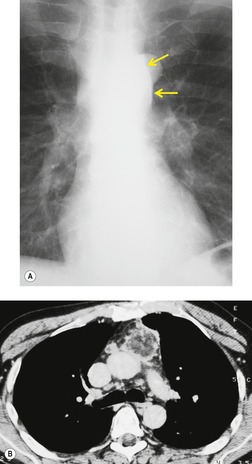
Thus, in HIV-infected patients with CD4+ lymphocyte counts above 200 cells/µL including those receiving ART, tuberculosis usually manifests on chest radiographs or CT with a postprimary pattern of disease: upper lobe linear and nodular opacities and cavitation (Fig. 6.5). 64 With more advanced degrees of immune suppression (CD4+ <200 cells/µL), cavitation becomes less common and disease increasingly manifests with focal or multifocal lobar or segmental opacities (often in atypical locations), bronchial wall thickening and centrilobular nodules (endobronchial spread), miliary nodules, enlarged hilar or mediastinal lymph nodes, and/or pleural effusion (Fig. 6.6, Fig. 6.7 and Fig. 6.8).55.56.57.58. and 59.,67.68. and 69. Lymphadenopathy may be the dominant imaging feature in some; mycobacterial infection is a common cause of isolated lymphadenopathy on chest radiographs in HIV-infected patients (Fig. 6.7). 60 Tuberculous lymph nodes frequently contain low attenuation centers and show rim enhancement (Fig. 6.7).70.71.72. and 73. CT has a limited role in the evaluation of HIV-infected patients with tuberculosis. CT can be used to: confirm the presence of parenchymal abnormalities suspected on chest radiographs; to further characterize abnormalities such as miliary or endobronchial spread of disease (Fig. 6.8); to identify cavitation or other complications of infection; and to evaluate suspected hilar or mediastinal lymphadenopathy (Fig. 6.7).
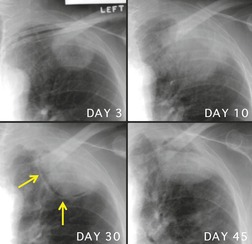
After diagnosis and institution of appropriate antituberculous therapy, serial radiographs usually demonstrate rapid clearing within months, with no evidence of residual disease. 74 Transient worsening of imaging findings may occur after institution of ART (Fig. 6.9); 75 improvement usually occurs within several weeks, however. If there is no clinical and radiographic improvement on appropriate treatment, concurrent opportunistic infection, tumor, or multidrug resistant tuberculosis should be considered. Noncompliance with antituberculous medications is also a problem in this population and is a further cause of treatment failure. Directly observed therapy may be required in this setting.76.77. and 78.
 |
| Fig. 6.9 (With permission from Haramati LB, Choi Y, Widrow CA, et al. Isolated lymphadenopathy on chest radiographs of HIV-infected patients. Clin Radiol 1996;51:345–349.) |
Nontuberculous (atypical) mycobacterial infection
Nontuberculous mycobacteria (NTM) are present worldwide, being found particularly in water and soil. 79 Although much less common than tuberculosis, NTM infection in HIV-infected individuals remains an important cause of morbidity and mortality in patients with advanced degrees of immunosuppression. 80 MAC and M. kansasii are the two most common pathogens in this setting (see Box 6.4). 1,9,81 In immune-competent patients, NTM disease is usually confined to the chest; in AIDS patients, disease is more often extrathoracic, disseminated and commonly involves gut, liver, lymph nodes, and bone marrow. 1 Infection confined to the thorax is rare in AIDS patients, but has been reported. 82 Infection due to MAC is thought to enter via the gastrointestinal tract causing systemic upset and chronic diarrhea; clinically significant pneumonia is uncommon in the AIDS population. 83,84 Organisms may be found in bronchoalveolar lavage fluid or sputum, blood, bone marrow, stool, and urine cultures; culture of the organism does not always correlate with clinical disease, however.
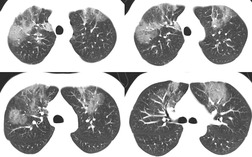
The chest radiograph is normal in most AIDS patients with disseminated MAC disease, even when the organism is cultured from respiratory secretions. 85 When radiographic findings are present (Figs 6.10 and 6.11), the predominant features are hilar and mediastinal lymphadenopathy and diffuse pulmonary opacities that often have upper lung predominance. 83,86,87 Less frequent manifestations include intrapulmonary nodules and pleural effusions, 86 endobronchial masses, possibly from erosion by hilar lymph nodes, and, rarely, cavitary upper lobe disease (Fig. 6.11). 83 CT, particularly high-resolution CT (HRCT), may demonstrate ground-glass opacities and bronchial wall and interlobular septal thickening. 87,88
The second most common atypical mycobacterium, M. kansasii, has a greater predilection for the lungs and causes disease that is clinically similar to infection with M. tuberculosis. A variety of radiographic appearances are found in AIDS patients with M. kansasii infection, including focal homogeneous segmental or lobar opacities (often in mid- and lower long zones) that may cavitate, focal or diffuse nodular or reticulonodular opacities, and thin-walled cavities.89.90. and 91. Pleural effusions are uncommon. 89 Intrathoracic lymphadenopathy is seen in 25% of patients and lymphadenopathy may be the only radiographic finding of infection in 12%.
The less common atypical mycobacteria, including M. gordonae, M. xenopi, M. fortuitum, M. chelonae and M. malmoense (Fig. 6.12) may also cause symptomatic pulmonary disease in HIV-infected patients. 92,93 A variety of radiographic patterns have been described with these infections, including interstitial opacities, scattered peribronchial opacities, miliary nodules, and parenchymal consolidation. 94,95
Fungal infection
Fungi are ubiquitous and commonly cause recurrent symptoms during the course of AIDS-related illnesses because efficient eradication of fungi requires a competent cell-mediated immune response.
Pneumocystis jirovecii (see Box 6.5)
The organism that causes Pneumocystis pneumonia in humans, formerly known as P. carinii, was renamed P. jirovecii in order to better reflect its distinctiveness from the organism that infects animals. 96 Further, although previously thought to be a protozoan, the organism is now regarded as a fungus. 96 Because of the confusion attendant to this change in nomenclature, the abbreviation PCP is still commonly accepted for the pneumonia caused by P. jirovecii.
Box 6.5
• The organism is now classified as fungus
• AIDS-defining illness
• Less common in patients receiving ART
• May still present in patients whose HIV status is unknown
• CD4+ lymphocyte count <200 cells/µL
• Common manifestations
– Perihilar hazy or ground-glass opacities
– Scattered thin-walled lung cysts
– Radiograph normal in up to 10%
– Spontaneous pneumothorax
• Uncommon manifestations
– Focal nodules or masses
– Focal consolidation
– Pleural effusion
– Organizing pneumonia (on ART)
• CT
– Helpful in diagnostically challenging cases
– Ground-glass opacities, typically spare subpleural lung
– Reticulation
Prior to the widespread use of antibiotic prophylaxis and ART, P. jirovecii caused pneumonia in over half of all patients with AIDS at some point in the course of their disease. Many AIDS-related deaths were due to respiratory failure directly attributable to PCP. The overall incidence of symptomatic infection has declined due to effective prophylaxis and ART, earlier recognition and more effective therapy (see Box 6.2). 97 PCP is now a less common cause of hospitalization and morbidity in HIV-infected individuals receiving ART than bacterial pneumonia, but remains an important cause of morbidity and mortality in underserved populations. 9 Nevertheless, PCP remains the most common opportunistic pneumonia in HIV-infected persons, even in developed countries where it may be the first presenting illness in patients with previously unrecognized HIV infection. 1,98
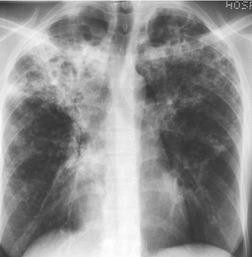
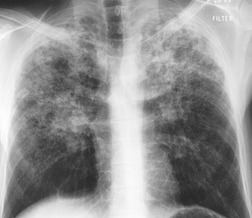
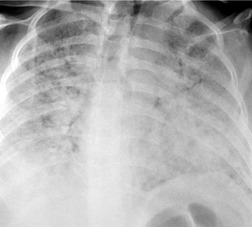
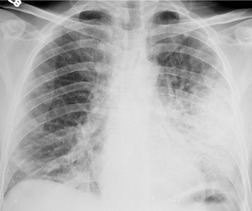
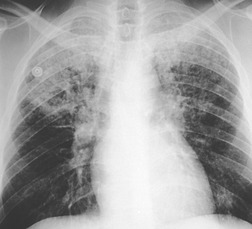
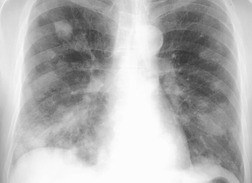
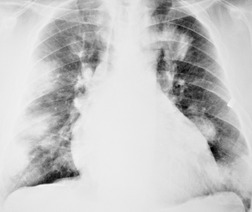
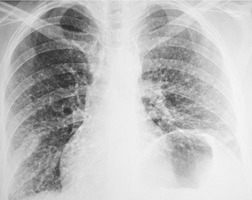
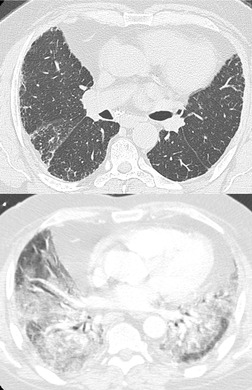
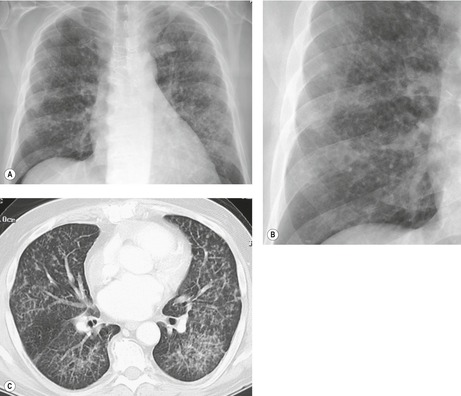
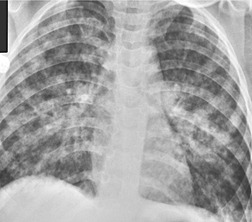
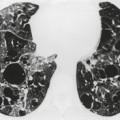
On chest radiographs, PCP typically manifests with diffuse or perihilar fine reticular and ill-defined ground-glass opacities (Fig. 6.13). 99 Untreated, these opacities may progress to diffuse homogeneous opacification in 3–4 days (Figs 6.14 and 6.15). Coarse reticular opacities may develop if infection persists (Fig. 6.16). 23,24,100,101 The chest radiograph is normal at presentation in up to 6% of symptomatic patients. 102 Hilar or mediastinal lymphadenopathy is rare, as is pleural fluid in the absence of extrapulmonary pneumocystosis.
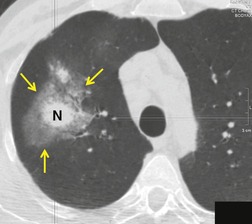
Atypical radiographic features are seen in 5–18% of cases of PCP. 100,103.104.105. and 106. Isolated segmental or lobar consolidation (Fig. 6.17) that can be mistaken for pyogenic pneumonia is occasionally seen. Focal nodular opacities with or without cavitation can occur (Fig. 6.18) and be confused with lung cancer (Fig. 6.19), lymphoma (Fig. 6.20), or metastases. 105,107 Such nodules may show granulomatous inflammation on histopathologic examination. 108,109
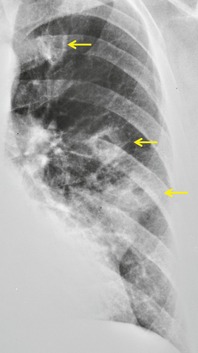 |
| Fig. 6.18 (Courtesy of Drs. P Needelman and B Suster, New York, NY, USA.) |
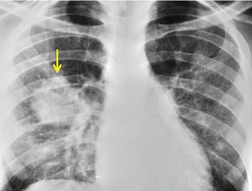 |
| Fig. 6.19 (Case courtesy of Drs. P Needelman and B Suster, New York, NY, USA.) |
PCP may rarely manifest as miliary nodules, pleural effusion, 103,104,110 endobronchial mass, or hilar and mediastinal lymphadenopathy, 100,103,104,106,111 which may be calcified.112.113. and 114. Upper lobe disease mimicking tuberculosis (Fig. 6.21) can be seen104,115 in patients using aerosolized pentamidine because of relative undertreatment of the lung apices. 115,116 Extrapulmonary disease involving the abdominal viscera is occasionally encountered. 112 Organizing pneumonia caused by P. jirovecii in the setting of IRIS has recently been described. 117
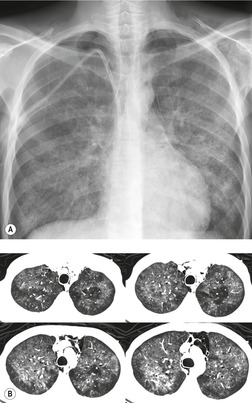
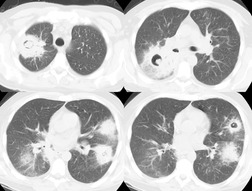
The CT and, in particular, the HRCT findings of PCP include scattered or diffuse ground-glass opacities, consolidation, and thickening of interlobular septa (Figs 6.13 and 6.14). 118,119 The CT findings may reflect disease chronicity with ground-glass opacities predominating in the early stages of the disease and with linear opacities predominating in cases of more chronic, repetitive, or undertreated infection (Fig. 6.16). 103 In practice, however, the disease affects both interstitium and airspaces in an unpredictable way; 120,121 thickening of interlobular septa can be the sole CT manifestation of PCP. 122 CT is usually not necessary in symptomatic patients with classic findings on chest radiographs. However, CT, particularly HRCT, can be quite useful for evaluating patients with suspected PCP and a normal chest radiograph or patients who have atypical radiographic findings for PCP. In this setting, the CT may show typical ground-glass opacities and be used to direct invasive diagnostic procedures (Figs. 6.22 and 6.23). 118,122.123.124. and 125. Conversely, a normal HRCT is good evidence that PCP is not the cause of symptoms. 122,124 Gallium-67 and DTPA radionuclide scans are sensitive but nonspecific for diagnosing PCP.126.127. and 128. Both are time consuming and expensive, may be poorly tolerated by debilitated patients, and are thus rarely used for this purpose.
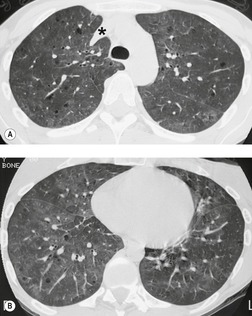
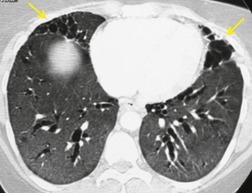
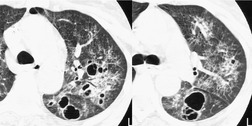
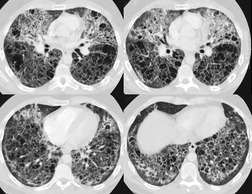
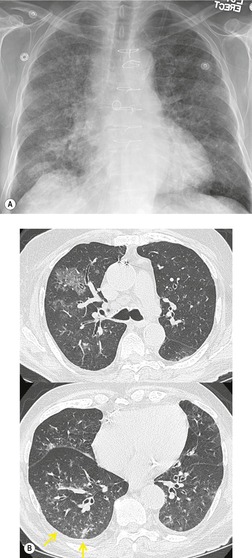
Up to one-third of patients with PCP develop thin-walled air-containing cysts (pneumatoceles), in either the acute or postinfective period (Fig. 6.24, Fig. 6.25, Fig. 6.26 and Fig. 6.27). 100,104,110,129,130 The incidence in one series of patients examined by CT was 38%. 103 The cysts are round, oval or crescent-shaped, and range in size from 1 cm to 8.5 cm; they are usually multiple but may be solitary. 109,129.130.131. and 132. They may occur at any location, although there was a predilection for the upper lobes in several series, 103,129,131,133 especially in patients on prophylactic aerosolized pentamidine (Fig. 6.24). 104,116 The etiology of Pneumocystis-related pneumatoceles is unclear, although a number of possible mechanisms have been suggested: 109,131,134,135P. jirovecii infection may stimulate macrophages to release elastase, tissue necrosis factor and other toxins that cause cystic destruction of lung parenchyma; vascular invasion by P. jirovecii may cause tissue necrosis and cavitation; and obstruction of small airways may lead to a ball valve effect on distal lung. A history of intravenous drug use136 and repeated prior infections are also likely to be associated with cystic change. The cysts usually resolve within 7 months of treatment, usually without sequelae. 130 However, cysts that persist up to 3 years have been reported. 137,138 Spontaneous pneumothorax due to cyst (Fig. 6.27) or subpleural bleb rupture may complicate the course of infected patients. 100,103,104,129,131,134,139.140. and 141.
Candida albicans
The yeast C. albicans is a normal commensal organism of the skin and mouth. In advanced cases of AIDS, it initially causes oral, then esophageal, candidiasis. 142 The incidence of candidal esophagitis does not seem to have decreased in the ART era. 142 Patients with esophageal candidiasis may present with severe, painful dysphagia. Barium swallow examination may show focal or diffuse irregular narrowing of the esophagus and diffuse ulceration. Although Candida is isolated from the airways or lungs of 2% of all patients with AIDS, 143 pulmonary candidiasis is a late, often preterminal, manifestation of HIV infection. It usually occurs in the setting of widely disseminated disease144 and nearly always occurs in AIDS patients who also have profound neutropenia. Histopathologic evidence of tissue invasion is required for definitive diagnosis of Candida pneumonia. In less severely immunocompromised patients, the organism may occasionally colonize preexisting tuberculous cavities or cystic spaces due to P. jirovecii infection. The imaging manifestations of pulmonary candidiasis are nonspecific and include diffuse heterogeneous or homogeneous pulmonary opacities. Nodules and thick-walled cavities are less common. 145
Cryptococcus neoformans
C. neoformans (see Box 6.6) is a ubiquitous fungus that infects up to 2% of patients with HIV infection and a markedly reduced CD4+ lymphocyte count (<100 cells/µL). 146 Cryptococcal infection in this population usually manifests with meningitis and disseminated disease. 1 Most cases occur in conjunction with other opportunistic infections; cryptococcosis is the sole manifestation of AIDS in less than 4% of patients. 143 Latent cryptococcal infection is believed to be an important cofactor for development of IRIS in patients receiving ART. 9,147
Box 6.6
• Cryptococcosis most common
– Diffuse reticulonodular opacities
– Lymphadenopathy
– Concomitant central nervous system (CNS) disease (e.g. meningitis) common
• Endemic fungi
– Disseminated histoplasmosis, coccidioidomycosis problematic
– CD4+ lymphocyte count <100 cells/µL
– Miliary disease, lymphadenopathy
• Aspergillosis
– Variety of forms
– Localized cavitary disease
– Disseminated disease (CD4+ lymphocyte count <100 cells/µL)
– Airway infection
– Obstructing bronchopulmonary aspergillosis
The clinical presentation of cryptococcal meningitis is often nonspecific. Patients may complain of vague symptoms and have a prolonged prodrome lasting from 1 to 4 months. Typical features of bacterial meningitis are usually absent. Less than half of patients complain of nausea and vomiting and only a quarter complain of neck stiffness or photophobia at diagnosis. The organism must be isolated from body fluids or tissues for definitive diagnosis. 143 The serologic test for cryptococcal capsular polysaccharide antigen is both sensitive and specific for invasive infection.
Cryptococcus accounts for 2–15% of all respiratory tract infections in patients with AIDS. 146 A variety of imaging patterns are described for pulmonary cryptococcal infection (Fig. 6.28, Fig. 6.29 and Fig. 6.30).148.149.150.151. and 152. Diffuse heterogeneous or reticulonodular opacities are common. 153 Infection can also manifest as solitary or multiple nodules varying from several millimeters to centimeters in diameter, with or without cavitation. Miliary disease, indistinguishable from tuberculosis, has been reported. 154 Intrathoracic lymphadenopathy is a common feature and may be the sole radiographic manifestation of disease. Pleural effusions are rare, occurring in less than 10% of cases in most series. Normal chest radiographs do not exclude the diagnosis. 152
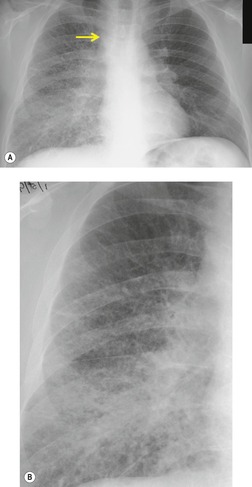 |
| Fig. 6.29 (With permission from Connolly JE Jr, McAdams HP, Erasmus JJ, et al. Opportunistic fungal pneumonia. J Thorac Imaging 1999;14:51–62.) |
 |
| Fig. 6.30 (With permission from Connolly JE Jr, McAdams HP, Erasmus JJ, et al. Opportunistic fungal pneumonia. J Thorac Imaging 1999;14:51–62.) |
Coccidioides immitis
C. immitis (see Box 6.6) is endemic in the semiarid regions of North America. It is widely distributed by the wind and then inhaled. 143 Symptoms are nonspecific and include night sweats, fatigue, and weight loss. Cough and dyspnea are reported in up to 50% of patients. Neurologic complications, typically meningoencephalitis or brain abscess, occur less commonly than in cases of cryptococcosis. When such complications occur, however, they are quite difficult to treat and have a very poor prognosis. Disseminated coccidioidomycosis usually occurs in the setting of low CD4+ lymphocyte counts (less than 200 cells/µL) and has very high mortality (up to 70%). 143,155,156 More localized pulmonary disease occurs in patients receiving ART. 156 Radiographs of affected patients show focal or diffuse homogeneous or heterogeneous pulmonary opacities. 157 Less common findings include discrete nodules, hilar or mediastinal lymphadenopathy, cavitation, and bilateral pleural effusions (1%).
Histoplasma capsulatum
H. capsulatum (see Box 6.6) is found throughout the world, but histoplasmosis is rare except in North America and the Caribbean. In immunocompetent individuals, it may cause a flulike illness that resolves spontaneously in almost all cases. In patients with impaired T cell function, progressive extrapulmonary infection leads to a systemic, debilitating febrile illness known as progressive disseminated histoplasmosis. Infection occurs in 2–5% of AIDS patients in endemic areas. 158 Affected patients complain of cough, dyspnea and weight loss, and 10% are frankly septic with lymphadenopathy and hepatosplenomegaly. 143 It is not clear whether pulmonary disease in AIDS patients results from reactivation of latent infection or from new exposure. Like tuberculosis, the intradermal skin test is negative in advanced disease and a positive antibody or prick test does not establish active current infection. 143 The highest diagnostic yields are from bronchoalveolar lavage samples or bone marrow aspiration and biopsy. 159 Identification of Histoplasma capsular polysaccharide antigen in blood and urine specimens is a rapid and relatively sensitive and specific means for diagnosing disseminated infection and for following patients during treatment.158.159.160.161. and 162. Latent disseminated histoplasmosis has been implicated as an important cofactor in development of IRIS in patients receiving ART. 163,164
Up to half of AIDS patients with disseminated histoplasmosis have normal chest radiographs. 165,166 Radiographs in the remainder may show diffuse small nodules (miliary pattern) (Fig. 6.31), linear opacities, and focal or diffuse homogeneous opacities. 165 Small pleural effusions and septal thickening may also be seen. On HRCT, the disease usually manifests with perivascular, paraseptal, and subpleural nodules as well as intra- and interlobular septal thickening – findings that are indistinguishable from those of miliary tuberculosis. 167 Mediastinal lymphadenopathy, which may be calcified, is noted in up to 13% of cases. 165,166 After treatment, parenchymal findings usually resolve completely. 165
Blastomyces dermatitidis
B. dermatitidis is endemic in North America and disseminated infection in patients with AIDS produces clinical features indistinguishable from other fungal infections or tuberculosis: notably fever, weight loss, cough, and dyspnea. 168 Typically, other concurrent AIDS-defining illnesses are present at the time of diagnosis and the CD4+ lymphocyte count is below 200 cells/µL. Disseminated blastomycosis is characterized by multiorgan involvement, including the central nervous system, and has high mortality (over 30%). Pulmonary involvement can lead to ARDS. There are very few reports regarding imaging manifestation of Blastomyces infection in AIDS patients. Pappas et al. 168 reported the chest radiographic manifestations of blastomycosis in 15 AIDS patients. In that series, a diffuse reticulonodular or fine nodular (miliary) pattern was most common. However, chest radiographs were normal in 27% of AIDS patients with disseminated blastomycosis.
Aspergillus
The incidence of Aspergillus infection (see Box 6.6) in HIV-infected patients remains fairly low in contrast to a high incidence (up to 41%) reported in patients with acute leukemia.169.170. and 171. This difference is because the neutrophil is the primary effector cell against Aspergillus. Thus, patients with HIV infection are not particularly susceptible to Aspergillus infection until they become very severely immunocompromised. Aspergillus infection usually occurs only when the CD4+ count falls below 50 cells/µL170.171.172. and 173. or when the patient becomes secondarily neutropenic due to use of bone-marrow suppressive drugs. Use of aerosolized pentamidine may be an independent risk factor. 173 Hemoptysis is a common and often fatal complication. 171
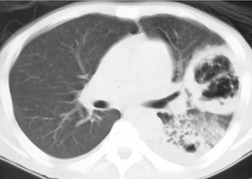
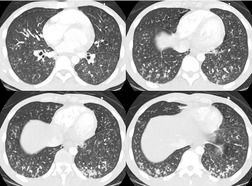
The radiographic manifestations of pulmonary aspergillosis in AIDS patients are quite varied, with several types of infection often present in the same patient. The most common pattern seen in the AIDS population resembles the type of infection described as chronic necrotizing aspergillosis. In this form, Aspergillus infection manifests with progressive cavitary pneumonia, usually in the upper lobes (Fig. 6.32). Cavitary lesions are reported in 20–80% of patients with AIDS and aspergillosis. 170,171,173.174.175. and 176. Intracavitary masses, due to either true mycetomas or infarcted lung, may develop. Noncavitating nodules, secondary to tissue invasion with or without infarction, have been reported in up to a third of cases (Fig. 6.33). 171,173,174,176 Invasive aspergillosis may also cause pulmonary consolidation and vascular invasion with infarction in AIDS patients. 170,171,173,175,176 Colonization of preexisting cavities or pneumatoceles may result in intracavitary aspergillomas. 174,177 Other reported features of aspergillosis in AIDS patients include pleural effusion, 173,174 pneumothorax, 170,171 lymphadenopathy, 173 cardiomegaly, 176 pericardial effusion, 176 and pericardial thickening. 174
There are several reports of a distinctive form of airway infection in AIDS patients known as obstructing bronchopulmonary aspergillosis. 171,175,178 In this unusual form of the disease, invasive airway infection leads to pseudomembranes and obstructing mucus plugs in the large airways. Chest radiographs of affected patients may be normal or show mucoid impaction, atelectasis, or obstructive pneumonia. 171,175 CT may show circumferential airway wall thickening, intramural sinus tracts, and large mucus plugs (Fig. 6.34). 178
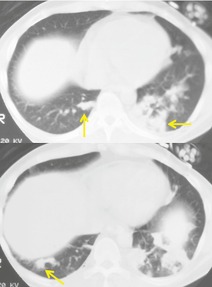 |
| Fig. 6.34 (With permission from Connolly JE Jr, McAdams HP, Erasmus JJ, et al. Opportunistic fungal pneumonia. J Thorac Imaging 1999;14:51–62.) |
Protozoal infection
Toxoplasma
The protozoan T. gondii is a frequent cause of focal neurologic abnormalities in patients with AIDS, but is an extremely rare cause of pulmonary infection. Nonspecific fever is the typical presenting manifestation when the thorax is affected. Occasionally, an acute disseminated form occurs that manifests with septic shock. 179 Affected patients are typically severely immunosuppressed with a mean CD4+ lymphocyte count of 32 cells/µL. 180 Diagnosis may be difficult because, in this setting, there may be no specific IgM Toxoplasma antibody response. Diagnosis is made by identifying the organism in bronchoalveolar lavage fluid or lung biopsy specimens. 180
Cryptosporidium
The protozoan Cryptosporidium is an unusual pathogen in the immunocompetent patient, being limited to farm workers or to individuals drinking contaminated water. In patients with AIDS, Cryptosporidium infection causes severe protracted diarrhea that is resistant to therapy. The organism passes from the gut into the biliary system and causes a form of sclerosing cholangitis. Pulmonary infection is quite uncommon, and, when it occurs, is thought to be due to aspiration of trophozoites during episodes of vomiting. Radiographic manifestations of Cryptosporidium pneumonia are quite nonspecific and include focal or diffuse air-space opacities that cannot be differentiated from other causes of pneumonia.
Human herpes virus infections
The herpes virus family of DNA viruses includes three viruses that can cause pneumonia in patients with HIV infection: CMV, herpes simplex, and varicella zoster.
CMV infection in immunocompetent adults typically results either in subclinical infection or in mild symptoms that resemble those of infectious mononucleosis. CMV infection in AIDS patients is usually more severe and manifests with retinitis, colitis, encephalitis, or radiculopathy. Over 90% of HIV-positive patients have latent infection, but clinically apparent disease emerges only when the CD4+ count is less than 60 cells/µL. The clinical significance of CMV as a pulmonary pathogen in AIDS patients is controversial182 as there are frequently many other concurrent pathogens, especially P. jirovecii.183 CMV pneumonia is generally considered quite uncommon in AIDS patients. 1 Most clinicians require evidence of CMV-related lung damage, such as cytomegalic inclusions in biopsy specimens, for definitive diagnosis. 184 Viral culture from respiratory secretions is considered less reliable evidence of infection. 185 The use of the newer antiretroviral drugs, in particular the protease inhibitors, is associated with a marked reduction in the development of CMV disease and an increase in survival for those with infection. 186
Very few reports have described the imaging manifestations of symptomatic CMV pneumonia in AIDS patients. The largest published series studied 21 patients in whom CMV was the sole pathogen. 182 Solitary or multiple nodules or masses, seen in 57% of cases, were the most common radiographic findings. The nodules varied in size from 2 mm to 3 cm in diameter. Pathologically, the lesions were due to focal areas of dense consolidation, alveolar injury, hemorrhage and fibrin collections, atypical necrotic lymphoid cells, or clusters of cytomegalic cells with focal necrosis. Ground-glass opacities were seen on CT in 43% of patients. Less common features included irregular linear opacities, bronchial wall thickening, bronchiectasis, and pleural effusion. Although the chest radiograph was normal in three patients, CT showed consolidation in two and ground-glass opacity in one. Endobronchial CMV causing circumferential narrowing and ulceration of the trachea has also been reported. 187
Failure of cell-mediated immunity in patients with AIDS allows reactivation of latent herpes simplex and varicella zoster infections and usually manifests with cutaneous eruptions. Pneumonitis is less common. The radiographic manifestations of pulmonary infection with these organisms are similar to those of CMV pneumonia: focal or diffuse homogeneous consolidation, diffuse heterogeneous opacities, or multiple lung nodules.
AIDS-associated airway disease and emphysema
Bronchiectasis has been reported in both HIV-positive and AIDS patients188.189.190.191. and 192. and is attributed to inflammation of the airways caused by P. jirovecii infection or recurrent pyogenic pneumonia (Fig. 6.35). 118 As discussed below, bronchiectasis is also seen in children with AIDS and lymphoid interstitial pneumonia (LIP). Organizing pneumonia has also been reported in association with HIV infection and may represent a response to undiagnosed infection. 191,193 There is an accumulating body of evidence that HIV infection predisposes to accelerated emphysema. 14,15
Immune restoration inflammatory syndrome
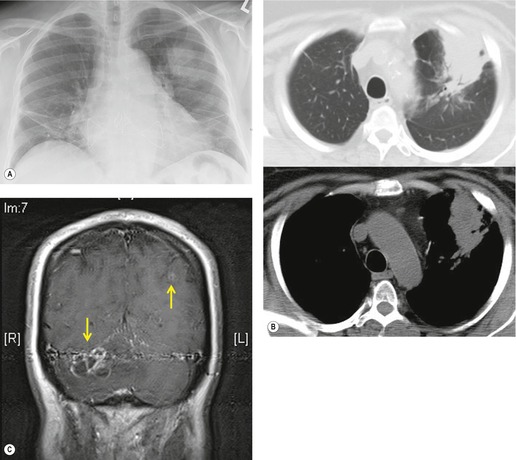
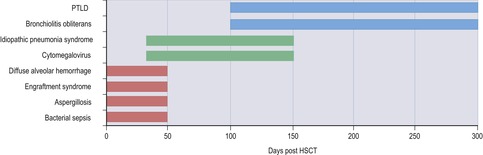
In recent years, the morbidity and mortality of HIV infection has dramatically declined, due in no small part to the introduction of so-called highly active ART. 6,194.195. and 196. Patients receiving ART have both a qualitative and quantitative improvement in T cell function, as reflected by the CD4+ lymphocyte count. 6,195 However, as the immune system reconstitutes, clinical and radiographic manifestations of previously latent opportunistic infections may become apparent, a phenomenon known as the immune restoration inflammatory syndrome (IRIS).3.4. and 5.,7,8,17,18,194,196.197.198. and 199. The reported incidence of IRIS varies from 3.6%196 to 32%200 of AIDS patients treated with ART. The clinical and imaging features of IRIS are thought to be the result of renewed potency of the immune response to latent subclinical infection, particularly to mycobacteria, fungi, and viruses. 7,18,201 Though the disease is frequently self-limited, antimicrobial therapy may be required. Fatal cases, though rare, are reported, particularly with CNS disease. 197 Affected patients usually begin to exhibit signs and symptoms of infection within 3 months of institution of the ART, 196 as the CD4+ lymphocyte count rises. Radiographic findings of disease are similar to those reported for these infections in the non-AIDS population, including mediastinal lymphadenopathy (Fig. 6.36), lytic bone lesions, localized abscesses, and parenchymal lung disease. 3,5,7,8,194,196,198,199,201
Typical clinical, imaging, and histopathologic features of sarcoidosis have also been reported in AIDS patients after beginning ART.202.203. and 204. It is thus possible that sarcoidosis represents a distinct manifestation of IRIS. Furthermore, transient worsening of existing clinical infection, particularly tuberculosis and PCP, has also been reported following initiation of ART. 5,205
Thoracic neoplasms and noninfectious complications
Kaposi sarcoma and non-Hodgkin lymphoma are the major neoplasms encountered in patients with AIDS and are probably mediated by viral infection. They may therefore be considered a complication of opportunistic infection. It is also possible that decreased cytotoxic T cell and natural killer cell function may contribute to development of neoplasms in patients with HIV infection.
AIDS-related lymphoma
Non-Hodgkin rather than Hodgkin lymphoma predominates in patients with AIDS (see Box 6.7), being invariably of high grade and usually of B cell or non-B–non-T cell origin. 206 The disease is typically extranodal and is in most cases associated with Epstein–Barr virus (EBV) infection. 207 EBV-driven polyclonal B cell proliferations, similar to those seen in organ transplant patients, and body cavity or primary effusion lymphomas associated with the human herpes virus type 8 (HHV-8) have also been reported in patients with HIV infection. 12,208
Box 6.7
AIDS-defining malignancies
Kaposi sarcoma
• Related to HHV-8 infection
• Incidence decreased in patients receiving ART
• Most with lung disease also have mucocutaenous lesions
• Imaging findings
– Peribronchovascular nodules, consolidation
– ‘Flame’-shaped nodules, air bronchogram sign
– Pleural effusion
Non-Hodgkin lymphoma
• Some related to EBV infection
• Incidence probably unchanged in patients receiving ART (controversial)
• Improved prognosis in patients receiving ART
• Extranodal disease (lung, pleura) common
Non-AIDS-defining malignancies
Lung cancer
• Usually nonsmall cell histopathology
• Increased incidence, even in (?because of) patients receiving ART
• Advanced stage at presentation, aggressive behavior12
The effect of ART on the incidence of AIDS-related lymphoma is controversial (see Box 6.2). 12 Some early reports suggested a decreased incidence, while later reports have found no significant change in incidence or even an increase, attributed to longer patient survival. 9,12 However, despite a relatively unchanged incidence, survival does appear to be improved in patients receiving ART. 12
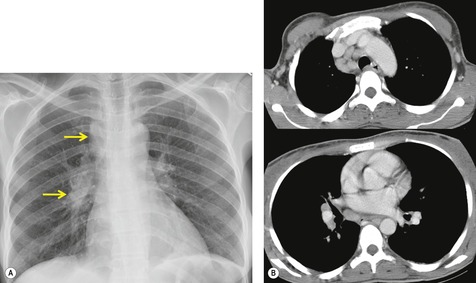
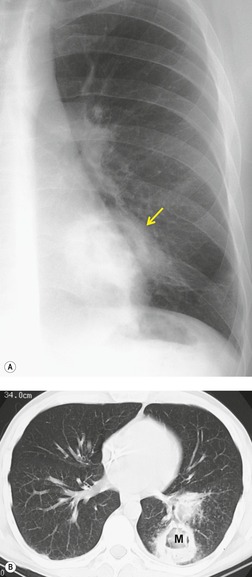
Thoracic involvement is reported in up to 40% of patients with AIDS-related lymphoma. 209,210 Extranodal pulmonary involvement in the setting of disseminated disease is the usual pattern, but primary pulmonary lymphoma has been reported. 211,212 The presence of extranodal disease predicts a poor outcome, with most affected patients surviving less than 1 year. 213 Parenchymal lymphoma usually manifests with solitary or multiple lung nodules or masses of varying size (Figs 6.37 and 6.38).209.210. and 211.,214,215 Cavitation is rare; one case of cavitation with an intracavitary mass resembling mycetoma has been reported. 209
Pleural fluid is also common in patients with thoracic AIDS-related lymphoma. Effusions may be unilateral or bilateral and are usually moderate to large in size.209.210. and 211. The pleura is sometimes the only site of disease (Fig. 6.39). 210 Unlike NHL that occurs in non-HIV-infected individuals, mediastinal or hilar lymphadenopathy is often a prominent imaging feature in AIDS-related lymphoma. Although reported in up to 45% of cases, adenopathy is usually not bulky or always multicompartmental. The nodes are often less than 1 cm in diameter and may therefore be seen only on CT.209.210. and 211.,216
Kaposi sarcoma
Prior to the advent of the AIDS epidemic, Kaposi sarcoma (see Box 6.7) was a rare neoplasm seen primarily in endemic form in sub-Saharan Africa, in elderly people in the Eastern Mediterranean and in heavily immunosuppressed patients. It had a relatively benign course and caused purple nodular lesions on the skin of the lower limbs; other forms of the disease were quite rare. 217 In patients with AIDS, Kaposi sarcoma is a multifocal polyclonal neoplasm with distinct histopathologic features. It is composed of a proliferation of vascular or lymphatic endothelial cells with associated spindle cells and atypical mitotic nuclear figures. The tumor forms multiple slitlike spaces that trap erythrocytes, a feature that accounts for the purple coloration of the lesion.
Kaposi sarcoma in AIDS patients particularly affects homosexual or bisexual men and individuals from Africa. It is uncommon in patients who acquire HIV infection transplacentally, through needle sharing, or from blood products. The incidence has decreased since the disease was first described in AIDS patients; this reduction occurred at the same time as the increased practice of safe sex and is presumed to be due to a decrease in the sexually transmitted form of the disease. Also, the incidence of Kaposi sarcoma is unequivocally decreased in HIV-infected patients receiving ART. 12 A close association has been documented between HHV-8 infection and Kaposi sarcoma. 218 The great majority of affected AIDS patients have cutaneous disease and over half also have oropharyngeal lesions; 219 a much smaller proportion have bronchopulmonary Kaposi sarcoma. 220 The clinical manifestations of pulmonary involvement are often nonspecific. Affected patients usually have advanced AIDS with a median CD4+ count of 34 cells/µL219 and present with fever and recurrent pneumonia. In most cases, cutaneous findings of Kaposi sarcoma precede visceral involvement.
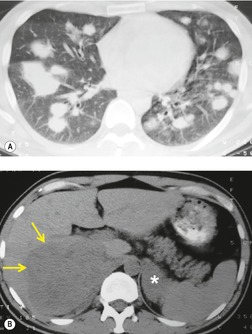
On chest radiographs and CT, focal Kaposi sarcoma may cause segmental, lobar-shaped, or masslike opacities due to the tumor itself (Fig. 6.40). Endobronchial disease may cause atelectasis or postobstructive pneumonia. 221 Disseminated pulmonary disease is seen more frequently. This form of the disease is distinctly bronchocentric in distribution as the lesions spread via the bronchial mucosa. Chest radiographs typically show nodular or coalescent masslike opacities in a perihilar and distinctively peribronchovascular distribution (Fig. 6.41). 220,222,223 On CT, 219,221,224.225. and 226. lesions are usually nodular in shape with either well- or ill-defined borders (Fig. 6.41, Fig. 6.42 and Fig. 6.43). Such lesions are sometimes described as ‘flame-shaped’ (Fig. 6.42). The lesions vary in size from less than 1 cm to well over 2 cm in diameter. The larger masses may contain air bronchograms (Fig. 6.43) and may have a surrounding rim of ground-glass opacity. Smooth or nodular thickening of bronchovascular bundles is seen in two-thirds of cases. Thickened interlobular septa and nodularity along the fissures are also common. 225 Up to half of affected patients have either unilateral or bilateral pleural effusions, which may be quite large. Hilar and mediastinal lymph node enlargement is seen in a substantial proportion of patients. While any of these findings may be seen in isolation, multiple findings are present in the great majority of patients. 224
 |
| Fig. 6.43 (Courtesy of Drs. P Needelman and B Suster, New York, NY, USA.) |
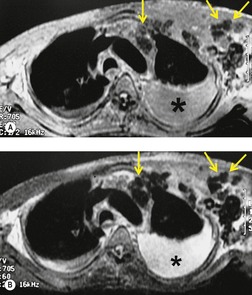
Magnetic resonance imaging (MRI) findings of Kaposi sarcoma were reported in a series of 10 cases. 227 The lesions were of high signal intensity on T1-weighted images and of low signal intensity on T2-weighted images. These findings were suggestive of hemorrhage within the lesions (Fig. 6.44). The masses also enhanced following intravenous administration of a gadolinium-based contrast agent.
Strictly speaking, either transbronchial or thoracoscopic lung biopsy is required for definitive diagnosis of pulmonary Kaposi sarcoma. Neither pleural fluid analysis nor pleural biopsy is diagnostic. In practice, however, the presence of typical imaging features (poorly defined nodules in a peribronchovascular distribution) and findings of endobronchial Kaposi sarcoma at bronchoscopy are regarded as evidence of pulmonary involvement.
Lymphoid interstitial pneumonia and other pulmonary lymphoproliferative disorders
Lymphocytic bronchiolitis, 228 lymphocytic alveolitis, 229 and LIP form a spectrum of atypical lymphoproliferative disorders encountered in patients with AIDS. Along with nonspecific interstitial pneumonia (see below), these are pulmonary complications of HIV infection that may occur in the absence of a detectable opportunistic infection or neoplasm. 230
LIP is an AIDS-defining illness in children less than 13 years of age. 231 LIP frequently occurs as part of a widespread syndrome of lymphocytic infiltration of parotid glands, stomach, thymus, liver, kidneys, and spleen. This syndrome is thought, in many cases, to be a reactive immune response to EBV. 207 It responds well to ART and has a relatively good prognosis. Although the radiographic features of LIP may persist unchanged for a long while, they may also resolve as the level of immunosuppression worsens. 232 Affected patients may be asymptomatic or present with subacute dyspnea and cough. Diagnosis in children rarely requires biopsy and is often based on the clinical and radiographic features alone. LIP in adults with AIDS is very uncommon and is thought to represent a specific pulmonary response to HIV infection. 233 Symptoms are nonspecific and include cough, dyspnea, and fever. 234 Histopathologic specimens show polyclonal proliferations of lymphocytes and micronodular lymphoid aggregates that diffusely infiltrate alveolar septa and bronchovascular bundles. 233,234
The imaging features of LIP are similar to, and often indistinguishable from, those of other infectious and noninfectious conditions that occur in patients with AIDS. Reticulonodular opacities are the most common finding on chest radiographs; the nodules vary from 1 mm to 5 mm in size (Figs 6.45 and 6.46).233.234.235. and 236. Lobar or segmental homogeneous opacities have also been described. 233 CT, especially HRCT, typically confirms the presence of small nodules in a diffuse distribution. Ground-glass opacities, bronchial wall thickening, and findings of bronchiolitis may also be seen. 235 Bronchiectasis, observed in children with LIP, 192 probably results from lymphocytic infiltration of the bronchioles leading to wall thickening, destruction, fibrosis, and dilatation. 237 CT may also demonstrate small, thin-walled air-filled cysts in cases of LIP. 238 The cysts may be related to partial obstruction of bronchioles from lymphocytic infiltration. Lymphadenopathy is seen in 25% of cases. 233 Gallium scanning may be positive, but is rarely performed. 239
Nonspecific interstitial pneumonia
Nonspecific interstitial pneumonia (NSIP), a noninfectious pneumonia that may be clinically indistinguishable from PCP, has been described in patients with AIDS. 230,241.242.243. and 244. Affected patients present with cough, dyspnea, and pyrexia. Histopathologic specimens show diffuse alveolar damage, an increased number of alveolar macrophages, alveolar hemorrhage, interstitial inflammation, and fibrin deposition. No causative agent has been identified, although patients may have a recent history of PCP, Kaposi sarcoma, drug therapy, or drug misuse.
The reported incidence of NSIP in patients with AIDS varies. One group evaluated lung biopsy specimens from 351 HIV-positive patients with presumed PCP. 243 Of the 67 patients who did not have PCP, 16 (24%) had findings of NSIP. Clinical symptoms, physical examination findings, and blood gas values were similar in patients with NSIP and patients with PCP. Patients with NSIP tended to present earlier in the course of their disease and had higher CD4+ lymphocyte counts (492 cells/µL versus 57 cells/µL) than patients with PCP. These authors concluded that NSIP may be the most common entity that mimics PCP in AIDS patients. They also found that NSIP tended to improve during empiric therapy for PCP. Another group evaluated 23 consecutive HIV-positive patients with no pulmonary symptoms, normal chest radiographs, no history of PCP, and no prior treatment with anti-Pneumocystis prophylaxis. 244 None of the patients had evidence of P. jirovecii or other infectious pathogens by stains of bronchoalveolar lavage fluid and histopathologic examination of lung biopsy specimens. However, transbronchial lung biopsy specimens showed findings of NSIP in 11 of 23 (48%) patients. It would thus appear that NSIP can exactly mimic the clinical features of PCP or be found by transbronchial lung biopsy in asymptomatic AIDS patients with normal chest radiographs.
Over half of symptomatic AIDS patients with biopsy-proven NSIP have a normal chest radiograph. Diffuse reticular opacities are noted in the remainder. 241 Radiographic findings vary in severity from minimal perihilar opacities to severe bilateral pulmonary consolidation, frequently associated with pleural effusion. Although the disease usually resolves without sequelae, repeated episodes may cause permanent lung injury and may contribute to long-term pulmonary dysfunction in patients with AIDS.
Persistent generalized lymphadenopathy
Persistent generalized lymphadenopathy is a feature of early but otherwise asymptomatic HIV infection. It characteristically affects the peripheral lymph nodes and, although documented in the nasopharyngeal lymphoid tissue, rarely causes important hilar or mediastinal lymph node enlargement.
Lung cancer
Lung cancer (see Box 6.7) is a common neoplasm encountered in HIV-positive patients. 12,245.246.247.248.249. and 250. Many, 247,249,250 but not all, 248 investigators have reported an increased incidence of lung cancer in AIDS patients, especially among women. However, whether the incidence of lung cancer in patients with HIV infection is truly increased or not continues to be debated. 12,250.251. and 252. The risk of cancer does not seem to correlate with CD4+ lymphocyte count. 9 ART has not yet seemed to affect incidence or prognosis; paradoxically, incidence may be increasing when compared with non-ART groups, perhaps because of longer survival. 9,12
Patients with AIDS and lung cancer are typically smokers, with an average age at diagnosis of approximately 40 years. Lung cancer frequently occurs early in the course of disease, sometimes before seropositivity for HIV infection is known. Rapid progression of carcinoma is the rule and the prognosis is extremely poor, with an increased proportion of poorly differentiated and advanced stage carcinomas. 253 The lung cancers are predominantly found in the upper lobes246,254 and have frequently spread to lymph nodes in the adjacent mediastinum or hilum (Fig. 6.47). 253 Extensive pleural disease from adenocarcinoma was present in 35% of HIV-positive patients with lung cancer in one study. 246
Pulmonary hypertension
There is an accumulating body of evidence showing that HIV-infected individuals are at increased risk of developing pulmonary hypertension. 1,9,16 While the precise etiology and pathogenesis for pulmonary hypertension in this population is debated, it may be related to HHV-8 infection. 1,16 As is the case in non-HIV-infected individuals, pulmonary hypertension in this population may be progressive and lead to respiratory failure.
Imaging diagnosis of pulmonary complications of HIV/AIDS
There is considerable overlap in the imaging manifestations of the various pulmonary complications of HIV/AIDS. Furthermore, the chest radiograph may be normal even when a pulmonary complication has developed. However, by carefully correlating clinical, laboratory, and radiographic findings with results of sputum analysis, bronchoalveolar lavage, or transbronchial biopsy results, a confident diagnosis can be made in most cases. 20,145 CT can be useful for either diagnosing or excluding pulmonary complications in symptomatic patients with normal or equivocal chest radiographs. 122,167,182 CT may also help limit the differential diagnosis when the chest radiograph is abnormal. 20,145,255.256.257. and 258.
Tuberculosis, bacterial pneumonia, PCP, Kaposi sarcoma, AIDS-related lymphoma, and septic emboli are the disorders most likely to be confidently diagnosed by chest radiography (see Table 6.1 and Box 6.8) or CT (see Box 6.9). The radiographic findings of other infectious or noninfectious complications are less specific and have extensive differential diagnoses. Several studies have investigated the diagnostic accuracy of chest radiography for specific AIDS-related pulmonary infections. 23,102,255 These studies show that findings of diffuse bilateral ground-glass, fine reticular, or air-space opacities are most likely due to PCP; however, other infections including bacterial pneumonia can rarely cause this pattern. Conversely, segmental or lobar consolidation suggests bacterial pneumonia; however, a small percentage of cases of PCP can manifest in this way. Scattered, dependent acinar nodules, particularly when seen in association with pulmonary cavities and enlarged hilar or mediastinal lymph nodes, strongly suggest tuberculosis. It should be noted that the diagnostic accuracy of chest radiography is significantly diminished when more than one complication is present. 255
| +, uncommon; ++, moderately common; +++, common; ++++, typical. | |||||||
| *Adapted from Haramati and Jenny-Avital.20 | |||||||
| Cause | Focal consolidation | Interstitial/nodules <5 mm | Nodules ≥5 mm | Cavity/cysts | Abnormal airways (CT) | Lymphadenopathy | Pleural effusions |
|---|---|---|---|---|---|---|---|
| Bacterial | |||||||
| Bacterial pneumonia | ++++ | – | + | ++ | – | + | ++ |
| Bronchitis, bronchiolitis | – | ++ | + | – | +++ | – | – |
| Mycobacterial | |||||||
| Tuberculosis | ++++ | ++ | +++ | ++ | ++ | +++ | +++ |
| MAC | + | ++ | ++ | ++ | + | ++++ | – |
| Fungal | |||||||
| Histoplasmosis | ++ | ++++ | ++ | ++ | – | ++ | ++ |
| Cryptococcosis | + | +++ | +++ | ++ | – | +++ | ++ |
| Aspergillosis | +++ | + | ++++ | ++++ | ++ | + | – |
| —Pneumocystis jirovecii | ++ | ++++ | + | +++ | – | – | – |
| Viral | + | +++ | ++ | + | ++ | + | + |
| Malignancies | |||||||
| Kaposi sarcoma | ++ | +++ | ++++ | – | +++ | ++ | +++ |
| Lymphoma | +++ | + | +++ | + | + | +++ | +++ |
| Lung cancer | ++++ | + | +++ | +++ | +++ | +++ | +++ |
Box 6.8
Focal opacities
• Bacteria
• Tuberculosis (high CD4+ count)
• PCP (low CD4+ count)
• Fungi
Diffuse opacities
• PCP (low CD4+ count)
• Tuberculosis (low CD4+ count)
• Kaposi sarcoma
• Bacteria
• Fungi
• CMV
Multiple nodules
• Kaposi sarcoma (‘flame-shaped’)
• Tuberculosis (miliary)
• Fungi
• Septic emboli
• Non-Hodgkin lymphoma
• Lung cancer
• LIP (children)
Cavities
• Tuberculosis (high CD4+ count)
• PCP (low CD4+ count)
• Pseudomonas pneumonia (low CD4+ count)
• Septic emboli
• Lung cancer
• Fungi
• R. equi
Pneumothorax
• PCP
Mediastinal lymphadenopathy
• Tuberculosis
• MAC
• Kaposi sarcoma
• Non-Hodgkin lymphoma
• Lung cancer
• Fungi
Pleural effusion
• Bacteria
• Tuberculosis
• Kaposi sarcoma
• Non-Hodgkin lymphoma
• Lung cancer
• Cardiomyopathy
*Adapted from Rosen.1
Box 6.9
• Scattered ground-glass opacities seen alone or separate from other abnormalities are suggestive of PCP
• Thin-walled cysts are a feature of PCP
• Multiple rounded irregular pulmonary nodules are suggestive of Kaposi sarcoma
• Solitary or multiple large rapidly growing pulmonary masses suggest AIDS-related lymphoma
• Irregular nodules in a peribronchovascular distribution suggest Kaposi sarcoma, but AIDS-related lymphoma should be considered
• Up to two-thirds of patients with known Kaposi sarcoma presenting with new pulmonary findings have coexisting opportunistic infection
• Low attenuation lymphadenopathy is suggestive of tuberculosis, particularly if there is rim enhancement
• Peripheral nodular, or wedge-shaped opacities with feeding vessels, cavitation, or extension into the pleural space are characteristic of septic emboli
*Adapted from Mason and Müller.257
Since these studies were limited to patients with single pulmonary complications and the observers were blinded to clinical and laboratory data, their results may not be directly applicable to everyday practice. This is certainly true for patients with more than one complication. Bearing these caveats in mind, Boiselle et al. 102 showed in a series of 163 patients with a variety of pulmonary complications that the accuracy of a confident imaging diagnosis was 84% for tuberculosis, 75% for PCP, and 64% for bacterial pneumonia.
OTHER FORMS OF IMMUNOCOMPROMISE
Pulmonary infection in immunocompromised patients
The ability of an individual to combat infections can be impaired in a variety of ways (see Box 6.10). The immunocompromised patient with fever and new pulmonary opacities is a very common clinical problem. Before the differential diagnosis is discussed in more detail, a few generalizations are applicable.259.260.261. and 262.
• New diffuse pulmonary opacities on chest radiographs in this population are associated with an overall mortality approaching 50%. Establishing the exact nature of the complication improves the outcome by no more than 10–20%. Even at autopsy, the exact diagnosis is not established in 15–20% of cases.
Box 6.10
• Neutropenia. Neutrophils are an essential line of defense against many microorganisms. Deficiencies in neutrophil numbers weaken host defenses. Neutropenia may be a feature of the disease itself or, more commonly, a consequence of drug or radiation therapy. The incidence of pulmonary infection rises steeply as the absolute neutrophil count falls below 1000 cells/µL
• Reduced cell-mediated immunity. T lymphocyte-dependent immune responses are an important line of defense, particularly against obligate intracellular parasites and ordinarily nonpathogenic commensal organisms in the respiratory tract
• Reduced humoral immunity. Impairment of B lymphocyte function reduces the antibody response to infective agents, diminishing host resistance. A reduction in circulating antibodies is a feature of various hypogammaglobulinemic states. Similarly, thymic aplasia, asplenia, or splenectomy can result in humoral immunodeficiency, leading to marked susceptibility to infection with encapsulated organisms such as S. pneumoniae
• Incompetence of cellular elements. An enzymatic defect in the neutrophils of patients with chronic granulomatous disease renders them incapable of combating certain microorganisms. Because silica particles can impair pulmonary macrophage function, patients with silicosis may not mount an adequate response to microorganisms such as M. tuberculosis or fungi263,264
• Nonspecific reduction in host resistance. Advanced age, alcoholism, diabetes mellitus, starvation or malnutrition, cancer, or other debilitating diseases can reduce an individual’s ability to combat infection
The chest radiograph yields a specific diagnosis in a minority of cases. In most instances, all that can be expected is a reasonable differential diagnosis that must be correlated with clinical and laboratory findings. Table 6.2, Table 6.3 and Table 6.4 outline conditions that should be considered when chest radiographs show segmental or lobar disease, diffuse lung disease, and nodular or masslike opacities respectively.
| *Solitary or multiple foci, with or without cavitation, with or without pleural fluid. | |||
| INFECTIOUS CAUSES | |||
|---|---|---|---|
| Gram-negative bacilli | Gram-positive bacilli | ||
| Klebsiella pneumoniae | Streptococcus pneumoniae | ||
| Serratia marcescens | Staphylococcus aureus | ||
| Escherichia coli | Proteus mirabilis | ||
| Pseudomonas aeruginosa | Haemophilus influenzae | ||
| Legionella pneumophila | |||
| Legionella micdadei | |||
| NONINFECTIOUS CAUSES | |||
| Pulmonary infarction | Lymphoproliferative disorders | ||
| *Respiratory syncytial virus, adenovirus, influenza, and parainfluenza viruses. | |||
| INFECTIOUS CAUSES | |||
|---|---|---|---|
| Protozoal | |||
| Strongyloides stercoralis | |||
| Mycobacteria | |||
| Mycobacterium tuberculosis | Non-tuberculous mycobacteria | ||
| Viruses | |||
| Cytomegalovirus | Varicella zoster virus | ||
| Herpes simplex virus | Respiratory viruses* | ||
| Fungi | |||
| Aspergillus species | Candida species | ||
| Histoplasma capsulatum | |||
| Pneumocystis jirovecii | |||
| NONINFECTIOUS CAUSES | |||
| Nonspecific interstitial pneumonia | Pulmonary edema Lymphangitic spread of tumor Leukemic infiltration | ||
| Drug reactions | |||
| Pulmonary hemorrhage | |||
| Radiation pneumonitis | |||
| INFECTIOUS CAUSES | |
|---|---|
| Nocardia asteroides | Legionella micdadei |
| Aspergillus species | Cryptococcus neoformans |
| Mucor species | |
| NONINFECTIOUS CAUSES | |
| Pulmonary infarction | Lymphoproliferative disorders |
| Neoplasm | |
Bacterial pneumonia
Bacteria are the most frequent cause of pneumonia in immunocompromised patients. In general terms, the clinical and radiographic features of pneumonia caused by organisms such as S. pneumoniae, Staphylococcus aureus, and P. aeruginosa do not differ from those seen in the general population. 265 Patients with neutropenia may show a slight lag in the appearance of pulmonary consolidation, and, in the group as a whole, pleural effusions and empyema are uncommon. On occasion, bacterial pneumonias become widely disseminated in the lungs of immunocompromised patients, something that is unusual in otherwise healthy individuals. Immunocompromised patients may be infected with bacteria almost never encountered in the general population such as R. equi.
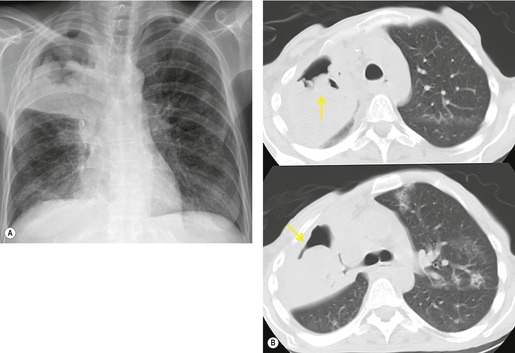
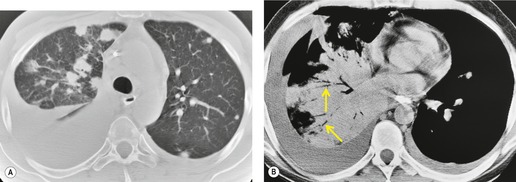
Patients receiving corticosteroid therapy and renal transplant recipients are particularly susceptible to Legionella organisms, including L. pneumophila and Legionella micdadei (Pittsburgh agent). 266 Urinary antigen detection is useful for rapid diagnosis of infection caused by L. pneumophila serogroup 1. 267L. pneumophila pneumonia is a rapidly progressive disease that begins with focal consolidation (Fig. 6.48) and may spread to involve both lungs diffusely. CT usually shows multifocal, bilateral, sharply demarcated peribronchovascular foci of consolidation and scattered ground-glass opacities. 268,269 Cavitation and pleural effusion are uncommon in the immunocompetent population, but are more common in immunocompromised patients. 37,270L. micdadei pneumonia is particularly associated with renal dialysis patients. It has a fairly characteristic radiographic pattern271 consisting of fairly well-circumscribed nodular densities (Fig. 6.49) with a distinct tendency to cavitate. The number of opacities is variable and the distribution may be widespread and random.
Tuberculosis and atypical mycobacterial infections, important pulmonary complications in AIDS patients, are uncommon in other immunocompromised patients. This presumably reflects the decline of tuberculosis in the general population. Reported cases probably represent reactivation of quiescent lesions; consequently, there is often a history of prior tuberculosis or a positive tuberculin skin test. Tuberculosis in non-AIDS immunocompromised patients is usually clinically and radiographically indistinguishable from postprimary tuberculosis. 272 On rare occasions, tuberculosis disseminates in a fulminant fashion, resulting in diffuse pulmonary disease. The radiographic features of tuberculosis are more thoroughly discussed in Chapter 5.
N. asteroides infection is fairly common in immunocompromised hosts, particularly in patients receiving corticosteroids and immunosuppressive agents (Fig. 6.50). The clinical presentation is subacute, and the radiographic abnormalities progress slowly. The radiographic features of N. asteroides infection are further discussed on page 247.
Fungal infection (see Box 6.11, Box 6.12, Box 6.13 and Box 6.14)
Aspergillus fumigatus is a rare cause of pneumonia in the general population but is an important pathogen in immunocompromised patients, particularly those with lymphoma or leukemia.273.274. and 275. Aspergillus infections are particularly problematic in patients with neutropenia or defects in neutrophil function such as chronic granulomatous disease. The pulmonary manifestations of aspergillosis form a broad spectrum of disease that ranges from allergic bronchopulmonary aspergillosis in hyperimmune hosts to mycetoma formation in preexisting lung cavities of otherwise normal patients (see p. 262) to invasive aspergillosis in immunocompromised hosts. 276 Overlap between these entities occurs; ‘semi-invasive’ or chronic necrotizing aspergillosis has been described in patients with mild degrees of immune dysfunction and underlying lung disease. 277 The following discussion is confined to the acute invasive forms of pulmonary aspergillosis that occur in severely immunocompromised patients: airway-invasive and angioinvasive aspergillosis (see Boxes 6.11 and 6.12).
Box 6.11
Infective agents
• A. fumigatus (most common)
• A. niger
Predisposing conditions
• Severe, prolonged neutropenia (<500 cells/µL)
• Hematologic malignancies
• Hematopoietic stem cell transplant recipients
• Chronic graft-versus-host disease
Clinical types
• Airway-invasive aspergillosis
• Angioinvasive aspergillosis
Clinical manifestations
• Cough, fever, chest pain, hemoptysis
• Progressive respiratory failure
• Mortality up to 70%
Box 6.12
Airway-invasive aspergillosis: CT findings
• Mucosal sinus tracts or fistulas in central airways
• Diffuse bronchial wall thickening
• Centrilobular nodules (‘tree-in-bud’)
• Peribronchial ground-glass opacity or consolidation
Angioinvasive aspergillosis: radiographic findings
• Initial: scattered poorly defined nodules or masses
– 1–3 cm diameter
– Few in number
• Late: more confluent opacities, cavitation (air crescent sign)
– Air crescent sign: late finding seen during neutrophil recovery
– Associated with good prognosis
Angioinvasive aspergillosis: CT findings
• Scattered nodules or masses with rims of ground-glass opacity (CT halo sign)
– Nodules >1 cm diameter, few in number
– Best seen by HRCT (partial volume effect)
– Most common, early finding
– May resolve by 1 week
• Less common
– Scattered or diffuse ground-glass opacities or consolidation
– Pleural fluid
• Focal, round hypoattenuating lesions with consolidation
– Later finding
– ‘Hypodense’ sign
• CT air cresent sign
– Late finding associated with recovery
Box 6.13
Infective agents
• Fungi of class Zygomycetes order Mucorales
• Genera: Rhizopus, Mucor, Absidia, Basidiobolus, Cunninghamella
Predisposing conditions
• Diabetes mellitus, especially with ketoacidosis
• Hematologic malignancies
• Solid organ transplant recipients
• Severe burns
• Deferoxamine therapy
Clinical types
• Rhinocerebral (most common)
• Pulmonary
• Abdominopelvic
• Cutaneous (burn patients)
• Disseminated
Clinical manifestations
• Occasionally asymptomatic in diabetic patients
• Cough, fever, chest pain, hemoptysis
• Progressive respiratory failure
• Mortality without aggressive early treatment approaches 80%
• Mimics invasive aspergillosis
• Resistant to voriconazole
• May develop in setting of voriconazole prophylaxis
Box 6.14
Radiographic findings
• Lobar or segmental consolidation, either unifocal or multifocal
• Solitary or multiple nodules
• Cavitation is frequent
• Air crescent sign occurs
• Pleural effusions
• Rapid progression
CT findings
As above with additional features in some cases:
• Air bronchograms
• Central low attenuation indicating necrosis
• Early detection of air crescent sign and cavitation
• Halo sign around nodules
• Endobronchial masses
• Hilar and mediastinal adenopathy
• Extrapulmonary invasion
• Pulmonary artery pseudoaneurysms
Airway infection by Aspergillus in neutropenic patients causes a necrotizing tracheobronchitis that can result in either focal or diffuse bronchopneumonia or, on occasion, even lobar pneumonia. 278,279 The disease is centered on the airways with direct invasion of the airway wall and adjacent lung. The findings on chest radiography and CT vary depending on the stage, severity, and extent of disease. In early stages, these examinations may be normal. 279,280 As disease progresses, either localized or diffuse heterogeneous or nodular opacities are seen on chest radiographs (Figs 6.51 and 6.52). CT usually shows airway-centered abnormalities consisting of bronchial wall thickening, peribronchial consolidation or ground-glass opacity, centrilobular nodules, and occasionally bronchiectasis (Fig. 6.52). 279 Sinus tracts in airway walls have also been described in patients with airway-invasive aspergillosis. Progressive infection may result in homogeneous segmental or lobar consolidation. Focal nodular opacities representing circumscribed areas of consolidation or abscess formation can also occur. 279,281
Angioinvasive aspergillosis, the most feared form of pulmonary aspergillosis, occurs in only the most severely immunocompromised patients. 275 Disease does not typically occur until the absolute neutrophil count is below 500 cells/µL for several weeks. Typical high-risk groups include allogeneic hematopoietic stem cell transplant (HSCT) recipients, patients with hematologic malignancies, patients receiving prolonged high-dose corticosteroid therapy, and patients with chronic graft-versus-host disease. While Aspergillus species are the most common cause of angioinvasive fungal pneumonia in immunocompromised patients, the incidence of pneumonia caused by other fungi including Candida species, Scedosporium species, Fusarium species and the zygomycetes (see below) may be increasing. 282 Angioinvasive fungal pneumonia in general, and aspergillosis in particular, is characterized by invasion of small and medium-sized blood vessel walls leading to in situ pulmonary infarction, hemorrhage, and ultimately systemic dissemination. Mortality remains high despite advances in early diagnosis and treatment; a favorable outcome often rests upon a high index of clinical suspicion, early detection of imaging (usually CT) findings, and institution of empirical therapy (see below). 275,282.283. and 284.
The typical clinical history is that of a severely neutropenic patient with fever, chest pain, and dyspnea. Initial chest radiographs are normal in up to one-third of cases. 280 CT can be very useful in this setting by demonstrating subtle ground-glass and nodular opacities (CT halo sign, see below) indicative of early infection opacities not evident on the chest radiograph.285.286.287.288. and 289. Eventually, however, chest radiographs will show one or more rounded areas of homogeneous opacification. The opacities are typically one to several centimeters in diameter, random in distribution, often peripheral in location, relatively few in number, and have poorly defined margins (Figs 6.53 and 6.54). 290 These opacities are due to a peculiar form of pulmonary infarction that occurs in cases of angioinvasive fungal infection – the so-called round or target infarction. 291,292 A round central region of coagulative necrosis, a rim of pulmonary hemorrhage, and a peripheral thrombosed pulmonary artery characterize this type of infarction. Later, with neutrophil recovery, an inflammatory reaction is found in the rim as well.
CT early in the course of infection demonstrates well the principal characteristics of the target infarction: a central round nodule or mass with a surrounding rim of ground-glass attenuation – the ‘CT halo sign’ (Fig. 6.53, Fig. 6.55 and Fig. 6.56). The ‘halo’ is due to hemorrhage around the infarct and is best seen with HRCT. The nodules are typically larger than 1 cm in diameter and, as noted above, relatively few in number. The halo sign is one of the earliest and most common CT findings of invasive aspergillosis and may be evanescent, resolving within a week of onset of infection. 285,293 Although the halo sign is a very consistent and early finding of invasive aspergillosis, it has been described in a variety of other infectious and noninfectious diseases.294.295. and 296. However, in the specific clinical setting of severe neutropenia and fever, the CT halo sign is very suggestive of early angioinvasive fungal infection. 287,293,296.297.298. and 299. Because prognosis is closely linked to early recognition and treatment, the CT halo sign plays a critical role for early diagnosis. In fact, studies have shown improved survival in patients who manifested the sign, perhaps because of early institution of treatment, compared with those who did not. 293 Other less common CT findings at initial presentation include smaller nodules, focal or multifocal consolidation, and wedge-shaped or tree-in-bud opacities. 293,296,298,300,301 Hilar or mediastinal lymphadenopathy is not a common feature of the disease and pleural effusions are generally seen only if hemorrhagic infarction results in bleeding into the pleural space. Invasion of chest wall or mediastinal structures is described but is quite rare. 302,303
Over the course of several days to weeks, the opacities tend to slowly enlarge and may coalesce into larger homogeneous, sometimes wedge-shaped, areas of consolidation (Fig. 6.57). Associated pulmonary hemorrhage can result in diffuse pulmonary opacification. On CT, focal well-defined round hypoattenuating areas (presumably again corresponding to the ‘target’ infarction) are seen within the areas of consolidation in up to 30% of cases (Fig. 6.57). 304 This finding has been termed the ‘hypodense’ sign and, in one study comparing a group of patients with invasive aspergillosis to a group with bacterial pneumonia, was specific for invasive aspergillosis. 304 After several weeks, the lesions may begin to cavitate. The earliest finding of cavitation is the so-called ‘air crescent sign’ in which a crescent of air develops along one margin of the lesion (Figs 6.54 and 6.58). 305 The lucency then enlarges to form a true cavity with an intracavitary mass (Figs 6.59 and 6.60). 306 The mass is due to necrotic lung and is usually adherent to the wall of the cavity – it is not typically gravity-dependent like a mycetoma. Cavitation is usually a late, not an early, finding of infection and occurs as neutrophil counts recover and the host begins to mount an immune response against the fungus. 307 Despite an appropriate clinical response to treatment, lesions tend to resolve fairly slowly. In one study of 40 treated patients followed by CT, the lesions enlarged in size and increased in number for several days (mean 9 days), but then the size and number reached a plateau and slowly resolved. Interestingly, less than 50% of patients showed complete remission at 80 days. Cavitary lesions tended to resolve more slowly than noncavitary lesions but were associated with a better outcome. 301
MRI has been used to investigate patients with angioinvasive aspergillosis. 308,309 The MRI characteristics depend on the age of the lesion. Early lesions, defined by Blum et al. 308 as being of less than 10 days’ duration, showed uniform low or intermediate signal intensity on T1-weighted images and high signal intensity on T2-weighted images. Early lesions uniformly enhanced with gadolinium-DTPA. There was no MRI correlate to the CT halo sign at this stage. With later evolution of the lesions, a targetlike appearance was seen on MRI consisting of a low-signal intensity center and an isointense or hyperintense rim on T1- and T2-weighted images. Areas of aging hemorrhage resulted in foci of hyperintensity within the low signal intensity center. 309 Gadolinium enhancement of the rim became manifest at this stage, presumably indicating an inflammatory reaction at the margin.
Disseminated aspergillosis may arise de novo or complicate the course of either pulmonary form. Pulmonary opacities in these patients range from a miliary to a more coarsely nodular pattern. 290,308 Hematogenous dissemination from a distant focus, as might be expected, results in diffusely distributed opacities in the lungs, whereas a heavy airway inoculum may give a more central, unevenly distributed pattern of bronchopneumonia.
Candida species may cause pneumonia alone, but more commonly do so in association with other fungal pathogens, particularly Aspergillus. 274 A significant proportion of patients with C. albicans pneumonia have leukemia or lymphoma, often in the later stages of treatment. 310 The majority of patients have widespread systemic dissemination of the organism. 311Candida pneumonia without systemic involvement is uncommon. 312 Diagnosis of C. albicans pneumonia is difficult, partly because pneumonia caused by other opportunistic organisms may also be present and partly because the presence of superficial colonization by Candida species may be discounted clinically. Typically the patient with disseminated candidiasis has severe and prolonged neutropenia, persistent fever despite antibacterial therapy, hepatomegaly, obvious colonies of C. albicans on the mucous membranes, and diffuse pulmonary opacities on chest radiographs. One group analyzed chest radiographic findings of 20 histopathologically proven cases of ‘pure’ pulmonary candidiasis. 310 Most cases were identified at autopsy, and the chest radiographs, obtained within 48 hours of death, presumably reflected advanced disease; all patients in this series died of disseminated candidiasis. Chest radiographs in nearly half the patients showed diffuse, bilateral, nonsegmental scattered nodular or heterogeneous opacities (Fig. 6.61). The remainder showed unilateral or bilateral lobar or segmental consolidation. Cavitation, adenopathy, masslike opacities, or diffuse miliary nodules were not observed. In contrast, Pagani and Libshitz313 described a pattern of diffuse miliary nodules in cases of pulmonary candidiasis. Another group failed to find any specific radiographic patterns in patients with pulmonary candidiasis, partly because of the high frequency of other pulmonary infections, edema, and hemorrhage. 314 On CT, the findings of candidal pneumonia are equally nonspecific: nodules and ground-glass and tree-in-bud opacities. 296,315 The CT halo sign is seen in up to one-third of cases. 315
Mucormycosis (also known as zygomycosis) is another common opportunistic fungal infection of the lungs (see Boxes 6.13 and 6.14). The infective agents belong to the class Zygomycetes, order Mucorales and include fungi of the genera Rhizopus, Mucor, Absidia, and Cunninghamella. 274 The most striking histopathologic feature of mucormycosis is vascular invasion resulting in infarction (angioinvasion, see above). 316,317 The diagnosis depends on identifying the organism by culture or by more directly showing histopathologic evidence of fungal invasion on biopsy. The fungal hyphae are broad, nonseptate, and branch at right angles. Furthermore, they can be readily seen on sections stained with hematoxylin and eosin. Diagnosis is critical because of the very high mortality in the absence of specific treatment with antifungal agents. Three groups of patients are particularly susceptible to mucormycosis: diabetics, patients undergoing treatment for hematologic malignancies, and recipients of solid organ transplants. Ketoacidosis is the critical predisposing factor in diabetic patients. Desferrioxamine therapy may also be associated with mucormycosis.
There are five clinical types of mucormycosis: rhinocerebral, pulmonary, abdominopelvic, cutaneous (seen primarily in burn patients), and disseminated. The pulmonary form is the second most common form after rhinocerebral mucormycosis. The imaging findings of pulmonary mucormycosis (Fig. 6.62, Fig. 6.63 and Fig. 6.64) are diverse. The disease most commonly manifests with either focal or multifocal pulmonary opacities that vary in shape from round to segmental or lobar. 318,319 Pulmonary mucormycosis tends to be localized in diabetic patients and has a relatively good prognosis. Conversely, mucormycosis in patients with hematologic malignancies tends to be multifocal or diffuse in distribution and carries a very poor prognosis. 320 The pulmonary opacities frequently cavitate. CT can show central low attenuation foreshadowing the development of cavitation. 320,321 Nodular opacities may show a halo sign on CT akin to that seen with invasive aspergillosis. 321 An air crescent sign may be seen in the initial stages of cavitation. 320 Intracavitary masses are common and are due to sloughed necrotic lung (Fig. 6.64). On occasion, the infection is so overwhelming as to cause pulmonary gangrene in which a large portion of lung sloughs into a thin-walled cavity. 322 Other CT features include endobronchial masses, hilar or mediastinal adenopathy, and pleural effusions. An unusual complication of mucormycosis is the development of multiple pulmonary artery pseudoaneurysms. 320,321,323 Extrapulmonary spread of disease to the spine and mediastinum is described (Fig. 6.62). Prompt diagnosis, usually with biopsy proof of disease, is essential because aggressive treatment can reduce the mortality rate from around 80% to as low as 25%. 320 In febrile neutropenic patients, mucormycosis often mimics the clinical and imaging findings of invasive aspergillosis, which is more common. Mucormycosis should be strongly considered in this setting, however, if disease is resistant to voriconazole.
 |
| Fig. 6.63 (With permission from McAdams HP, Rosado de Christenson M, Strollo DC, et al. Pulmonary mucormycosis: radiologic findings in 32 cases. AJR Am J Roentgenol 1997;168:1541–1548.) |
C. neoformans pneumonia occurs in immunocompromised patients, although the pneumonia is often overshadowed by cryptococcal meningitis. Cryptococcal pulmonary infection is discussed in detail on page 254.
Widespread dissemination of blastomycosis, coccidioidomycosis, and histoplasmosis (Fig. 6.65) occurs in immunocompromised patients and has serious consequences. However, given the frequency of these diseases in certain geographic areas it is surprising that these fungi are so rarely implicated as opportunistic infective agents. Specific diagnostic features are lacking, and the clinical thrust is likely to be toward excluding infection with the more common opportunistic fungi.
Pneumocystis jirovecii
As noted above, P. jirovecii is the new name for the organism previously known as P. carinii. It has also been variably classified as a parasite and as a fungus although recent evidence argues for a fungal origin. 98,324,325 And, as previously discussed, P. jirovecii remains the most common opportunistic agent in AIDS patients. 98 However, P. jirovecii is also an important pathogen in other groups of immunocompromised patients, including malnourished infants, children with primary immune deficiencies, organ transplant recipients, and patients being treated for inflammatory or collagen-vascular disorders, hematologic malignancies, or brain neoplasms (Fig. 6.66). 98,324.325.326.327.328.329.330.331.332. and 333. An important risk factor in many of these patients is prolonged, high-dose corticosteroid therapy, cytotoxic therapy, or, more recently, antitumor necrosis factor-α therapy. 333 In a study of 116 patients with PCP, there were 37 patients whose only cause of immune compromise was corticosteroid therapy for a variety of inflammatory conditions, including sarcoidosis and Wegener granulomatosis. 334 The median dose of steroids in these patients was 40 mg/day. Nevertheless, 25% of the patients were on half these doses for 8 weeks or less before diagnosis. Brain tumor patients receiving high-dose dexamethasone and concomitant chemotherapy are at particular risk for developing PCP. 327,329,331 Risk factors include duration of therapy greater than 5 weeks and lymphopenia. 327,329,331 Some authors have reported a particular propensity for pneumonia to occur during steroid taper. Patients with endogenous corticosteroid production (Cushing syndrome) are also at risk for PCP. 330
Diagnosis of PCP can be difficult because the entity may not be considered in non-AIDS patients. Also, many affected patients have conditions such as sarcoidosis, Wegener granulomatosis, or pulmonary drug toxicity, diseases whose radiographic manifestations could mask the appearance of PCP. The imaging features of PCP in non-AIDS patients are similar to those seen in AIDS patients, as discussed on page 302. However, PCP in this population can be more fulminant than is typically seen in AIDS patients, perhaps because the diagnosis is not suspected until later in the course of the disease. 333 Mortality up to 50% has been reported for PCP in the non-AIDS population. 326 Prompt diagnosis therefore requires knowledge of the important risk factors for PCP in non-AIDS patients and a high index of suspicion. The diagnosis should be strongly suspected in patients treated with high-dose corticosteroids, who develop dry cough, dyspnea, and hypoxemia, new bilateral opacities on chest radiographs, and geographic ground-glass opacities on CT (Fig. 6.66). 98,332
Viral infection
CMV is the most common viral pathogen in immunocompromised patients. Infection results from defects in cell-mediated immunity and most frequently occurs in patients with AIDS or hematologic malignancies or in organ transplant recipients. 335 Diagnosis is complicated by the fact that CMV infection is very common in the general population and many immunosuppressed patients are latent carriers of the virus. 185 Distinction of the carrier state from active infection is difficult. This diagnostic difficulty is further compounded by the fact that the clinical manifestations of infection are quite variable, ranging from completely asymptomatic to catastrophic infection. Furthermore, clinical symptoms and imaging features of infection are nonspecific and are often indistinguishable from other complications. CMV may also coexist with or even predispose to other pneumonias, especially PCP. 336 Rapid culture (shell-vial technique) of virus from respiratory secretions or biopsy material is considered suggestive of infection, as are positive CMV serologies. 185 However, demonstration of characteristic CMV cellular inclusion bodies is considered more definitive for diagnosis of active infection. 335 Even then a response to antiviral therapy is often required for full confirmation. Quantification of viral load by quantitative polymerase chain reaction (PCR) techniques is also helpful for deciding when to treat, and to identify high-risk patients for prophylactic therapy. 185
The imaging findings of CMV pneumonia are varied and overlap those of other opportunistic infections, particularly PCP. 332,337 On chest radiographs, CMV pneumonia most commonly manifests with bibasilar heterogeneous opacities, often with a slightly nodular character (Fig. 6.67). Although the opacities are usually bilateral, focal opacities mimicking bacterial pneumonia are occasionally seen, 338 as are pleural effusions. Less common manifestations include diffuse fine (miliary) nodular opacities. Spontaneous pneumothorax and pneumomediastinum can occur in cases of advanced CMV pneumonia. 337 The chest radiograph may also be normal despite biopsy-proven infection. The CT manifestations of CMV pneumonia are equally varied and include scattered or diffuse ground-glass opacities, nodules up to 5 mm in size, masses, consolidation, and reticular opacities. 182,339.340. and 341. Multiple small nodules, ground-glass opacities, and consolidation are the most suggestive findings on CT (Fig. 6.67). 339,341 Pleural fluid is common on CT. 340 CT in affected patients usually shows more extensive abnormalities than are seen radiographically. CT can also be normal in patients with biopsy-proven disease. 339 Because CMV pneumonia is often confused with PCP, one group of investigators compared CT findings in 31 cases of proven CMV pneumonia with those of 27 cases of PCP. 332 While there was significant overlap in the CT appearance of the two pneumonias, patients with CMV infection were more likely to have small nodules, nongeographic ground-glass opacities, and consolidation. Conversely, patients with PCP were more likely to have geographically distributed ground-glass opacities in an apical distribution. 332
Other viral pathogens capable of causing severe pneumonia, especially in patients with lymphoma, are the varicella zoster virus and the herpes simplex virus. Varicella zoster pneumonia is fulminant, with development of extensive scattered areas of parenchymal consolidation that may become widely confluent (Fig. 6.68). Diagnosis is facilitated if the patient has coincident disseminated herpes zoster or varicella.
Protozoal infection
S. stercoralis is a common intestinal parasite in many parts of the world. Infection confined to the intestinal tract may be asymptomatic or cause nonspecific complaints such as diarrhea, weight loss, or abdominal pain. 342 Severe compromise of the immune system, however, may lead to massive proliferation of the organism in the gastrointestinal tract. The filariform larva then penetrates the colonic wall or perianal skin and migrates to the lungs. An overwhelming burden of larvae may result in systemic dissemination, for example, to the CNS.342.343.344.345. and 346. This phenomenon is termed hyperinfection syndrome and is associated with high mortality, up to 87%. 342 Hyperinfection syndrome has been reported in a variety of settings, including HIV infection, after solid organ or hematopoietic stem cell transplantation, malnutrition, and as a manifestation of IRIS.342.343.344.345.346. and 347.
The chest radiograph in patients with Strongyloides hyperinfection syndrome may be normal or show widespread pulmonary opacities (Fig. 6.69).348.349.350. and 351. Eosinophilia, a feature of helminthic infections, is often present. The organisms may be detected in sputum, small bowel aspirates, or stool. 352 There may be an accompanying Gram-negative bacteremia, and this, together with the patient’s intestinal symptoms, may provide a clue to the diagnosis.
Imaging evaluation and differential diagnosis of pulmonary opacities in immunocompromised patients
Fever and new pulmonary opacities are commonly encountered problems in immunocompromised patients. Elucidation of their cause requires consideration of the following: cause of immune impairment; results of previous tuberculin testing; whether the infection was acquired in or out of hospital; whether there is any known potential source of infection; and the results of blood or sputum cultures and serologic testing.
Chest radiography is usually the first imaging study requested for these patients. Radiography is useful for detecting intrathoracic abnormalities, but is limited in its ability to delineate a precise etiology. In an autopsy study of patients with leukemia, chest radiography was deemed valuable for detecting pulmonary abnormalities, but, as noted, very limited in its ability to discriminate causes, with the exception of ARDS. 262 However, most of the 45 patients in this series were critically ill with more than one infectious or noninfectious complication; only seven patients had a single complication (either ARDS or pulmonary hemorrhage). In a less critically ill group of non-AIDS patients with a wide range of causes of immune compromise, the correct diagnosis was made on the basis of the chest radiograph in 34% of cases, a figure that seems high. 260 Winer-Muram et al., 261 in a study involving less critically ill patients with hematologic malignancies, had better results with chest radiography, particularly with fungal pneumonia and cryptogenic organizing pneumonia.
CT can play an important role in the evaluation of persistently febrile immune-suppressed patients. The two best-studied populations in this regard are patients with AIDS and severely neutropenic patients. As previously noted, CT, particularly HRCT, can be useful in AIDS patients with symptoms of pneumonia and a normal or equivocal chest radiograph. In this setting, CT can be useful for either diagnosing or excluding pulmonary complications such as PCP. 118,122.123.124. and 125.,167,182
In the febrile patient with neutropenia, CT can be useful for showing findings of pneumonia when the chest radiograph is normal or equivocal, for better characterizing the nature of radiographically evident abnormalities, and for planning diagnostic procedures such as fiberoptic bronchoscopy or bronchoalveolar lavage. 353,354 One group of investigators prospectively studied 87 patients with fever and neutropenia of more than 2 days’ duration despite empiric antibiotics. 355 Of the patients with a normal chest radiograph, almost half had findings suggestive of pneumonia on HRCT. In a separate study, the same group reported that CT showed findings of pneumonia in 60% of 112 persistently febrile neutropenic patients with normal chest radiographs. 356 In both studies, CT showed findings of pneumonia about 5 days earlier than did chest radiographs, leading the authors to conclude that all febrile neutropenic patients with normal chest radiographs should undergo CT. In another study of 33 febrile neutropenic patients, chest radiographs were interpreted as normal in one-third and as showing nonspecific findings in the remainder. 357 In this study, CT findings resulted in a change in clinical management in one-third of patients. Gulati et al. 358 assessed the utility of HRCT in 21 consecutive renal transplant recipients with suspected pulmonary infection. Compared with chest radiography, HRCT revealed additional findings suggestive of the correct diagnosis in 11 (50%) patients. CT has also been shown to be helpful for predicting the nature of pulmonary complications in febrile HSCT recipients. When CT shows nodular opacities, pulmonary fungal infection (usually aspergillosis) is usually the cause. 359 When CT is negative, bacteremia or nonpulmonary fungal infection is usually the cause. 359
Owing to the high incidence, morbidity, and mortality of invasive Aspergillus infection in neutropenic patients, many investigators have studied the use of CT for early diagnosis. 275,282,284 One group showed that CT was better than a variety of novel serologic tests for early diagnosis of aspergillosis in HSCT recipients. 360 In that series, CT showed abnormalities at least a week before these tests became positive. In two separate studies, systematic use of CT allowed very early recognition of Aspergillus infection in at-risk patients. 285,361 In one of the studies, mean time to diagnosis was reduced from 7 to 2 days. 361 They also reported that earlier diagnosis and aggressive management could improve outcome. 285,361 These findings have been reproduced by others. 275,282.283. and 284. Galactomannan (an antigen released when Aspergillus hyphae invade host tissue) assays performed on either serum or bronchoalveolar lavage has proven useful for diagnosis of invasive Aspergillus infection. 362,363 However, galactomannan positivity was found to lag, by at least several days, the development of characteristic CT findings in 107 patients with aspergillosis. 364 Galactomannan assays were also found less likely to be positive in cases of airway invasive aspergillosis (60%) than in cases of angioinvasive disease (80%). 365 CT and galactomannan assays are probably complementary diagnostic approaches and, when used appropriately, may facilitate early diagnosis and treatment of Aspergillus infection in immunocompromised hosts. 366
In summary, a wealth of data supports the role of CT for evaluation of febrile neutropenic patients who have normal or equivocal chest radiographs. In this setting, a normal CT suggests a nonpulmonary complication. An abnormal CT suggests a pulmonary complication, can suggest the nature of that complication, and can direct further invasive testing such as bronchoalveolar lavage or transbronchial biopsy. It should also be remembered that a host of noninfectious complications can cause pulmonary problems in immunocompromised patients. These include, but are not limited to, neoplastic involvement of the lung (see Chapter 13), drug or radiation-induced toxicity (see Chapter 9), transfusion reactions, pulmonary edema, and pulmonary hemorrhage. Transfusion reactions are usually acute and the temporal relationship to the transfusion of blood or blood products is clear. The reaction may simply be one of volume overload and pulmonary edema. More capricious are the agglutinin reactions resulting from an excess of antibodies in the donor serum directed against the recipient’s cells, particularly granulocytes. The result is the abrupt onset of fever, chills, tachypnea, and tachycardia coincident with the development of varying patterns of pulmonary edema (Fig. 6.70). The edema pattern may persist for 24–48 hours, and a response to corticosteroid therapy may occur. Leukemia may be associated with pulmonary hemorrhage. The clinical features may clearly indicate the bleeding tendency, and the abrupt appearance of scattered homogeneous opacities with hemoptysis and a fall in the hematocrit may allow confident diagnosis. The incidence of pulmonary hemorrhage in leukemia is difficult to ascertain, particularly because the coagulopathy may preclude biopsy. Pulmonary hemorrhage varying from microscopic to massive hemorrhage is seen in some three-quarters of leukemic patients at autopsy. 367 Pulmonary hemorrhage may be associated not only with a bleeding tendency, but also with infection or diffuse alveolar damage. 367,368 Tenholder and Hooper369 suggest that the incidence of pulmonary hemorrhage as a sole cause of pulmonary opacities in leukemic patients may be as high as 40%. This seems a high figure, but it does serve to emphasize that pulmonary hemorrhage is probably underdiagnosed.
Nonspecific interstitial pneumonia and organizing pneumonia
Exhaustive clinical investigation may fail to establish the etiology of pulmonary opacities in immunocompromised patients. Lung biopsy, either by video-assisted thoracoscopy or by fiberoptic bronchoscopy, may be required in the most problematic cases. In a considerable number of patients, however, histopathologic examination of affected lung only shows findings of diffuse alveolar damage, NSIP, or organizing pneumonia (OP); etiologic agents cannot be identified. NSIP is found in some 30–45% of biopsies in this clinical setting.370.371. and 372. It is possible that lung injury in these patients is multifactorial and may be a manifestation of undiagnosed infection, pulmonary drug toxicity, radiation therapy, sepsis, etc. Organizing pneumonia is probably less commonly encountered in this setting than NSIP but responds well to corticosteroids and has a more favorable prognosis (Fig. 6.71). The radiographic and CT findings of organizing pneumonia are discussed in detail on page 575. Because the imaging findings of OP are quite similar to those of other pulmonary complications in immunocompromised patients, the diagnosis is typically made at biopsy.373.374.375. and 376. Organizing pneumonia should be clearly distinguished from obliterative bronchiolitis, a distinct condition that results in irreversible airway occlusion in hematopoietic stem cell and lung transplant recipients. Transplant-related obliterative bronchiolitis generally has a very poor prognosis. 377
Hematopoietic stem cell transplantation
Many thousands of HSCTs (formerly called bone marrow transplants) are performed annually for a diverse range of malignant and nonmalignant conditions (see Box 6.15). 378,379 Pulmonary complications are seen in 40–60% of HSCT recipients and many of the complications (see Box 6.16) carry significant mortality. 380,381 In previous years, infection was the most common cause of pulmonary complications after HSCT. Now, because of advances in transplant protocols and procedures and more effective antimicrobial prophylaxis and treatment, noninfectious complications are the major cause of pulmonary morbidity and mortality after transplantation. 382,383 Important risk factors for development of pulmonary complications after HSCT include the pretransplant conditioning regimen, the type of transplant, the duration of immune system dysfunction, and the presence of graft-versus-host disease (GVHD). 384
Box 6.15
• Acute leukemia
• Chronic myelogenous leukemia
• Chronic lymphatic leukemia in younger patients
• Lymphoma – Hodgkin and non-Hodgkin
• Multiple myeloma
• Aplastic anemia
• Hemoglobinopathies – sickle cell disease, thalassemia
• Myelodysplastic syndrome
• Immunodeficiency disorders
• Solid tumors, e.g. breast, testicular cancers, neuroblastoma
• Scleroderma
• Miscellaneous genetic disorders, e.g. Gaucher disease, osteopetrosis
Box 6.16
Noninfectious complications
• Acute graft-versus-host disease
• Chronic graft-versus-host disease
• Interstitial pneumonia
• Pulmonary edema
• Pulmonary hemorrhage
• Acute tracheobronchitis
• Pulmonary venoocclusive disease
Infectious complications
• Viral, especially CMV
• Bacterial
• Fungal, especially Aspergillus species
• P. jirovecii
Most myeloablative stem cell transplant procedures involve infusion of progenitor stem cells into a patient whose bone marrow has been ablated by high-dose chemotherapy and total body irradiation (the conditioning regimen). Because the patient may receive radiation to the entire lungs and the chemotherapeutic doses used are often quite formidable, the conditioning regimen employed is a major factor contributing to pulmonary complications. In older patients or in those with poor cardiopulmonary reserve, ‘mini’ or nonmyeloablative transplant techniques that use more moderate conditioning regimens combined with posttransplant immunosuppression are sometimes employed. 384 Complications of these transplants are less severe than those of traditional myeloablative transplants, perhaps because of the more moderate pretransplant conditioning regimen. 385
Transplanted stem cells may be harvested from the patient prior to conditioning (autologous HSCT), from an identical twin (syngeneic HSCT), or from a human leukocyte antigen (HLA)-matched donor source (allogeneic HSCT). Common to all myeloablative transplant procedures is a 2–4-week period of profound bone marrow dysfunction between the time of stem cell infusion and engraftment. During this period, red blood cells and platelets can be supplied by transfusion, but the consequent neutropenia renders the patient susceptible to bacterial and fungal infection. Neutrophil production usually begins to accelerate after about 2 weeks and the period of severe neutropenia usually lasts only about 4 weeks. Neutropenia is only a part of the profound immune deficit in these patients, though. Lymphocytes, for example, are the most radiosensitive cellular element in the body and can also be depleted. Immune dysfunction is less severe and less prolonged in patients receiving autologous and nonmyeloablative transplants. Hence, their risk for opportunistic infection (especially CMV and fungal pneumonia) is reduced compared with patients receiving myeloablative allogeneic transplants. After the first month, there is a continuous recovery of immune function that is usually complete at about 1 year. Graft-versus-host disease (GVHD) results from transplantation of immunocompetent donor lymphocytes that attack the recipient’s tissues and can develop at any time after transplant. Recipients of autologous or syngeneic stem cells are, of course, not susceptible to GVHD. Both the development of, and treatment for, GVHD can predispose transplant recipients to further pulmonary complications, including infection.
The pulmonary complications of HSCT are customarily divided (see Fig. 6.72 and Box 6.17) into those that occur in the 30 days after transplant (neutropenic phase), those that occur between 30 and 100 days after transplant (early phase), and those that occur thereafter (late phase). The advantage of this temporal division is that certain complications, although their clinical and imaging manifestations may be similar, tend to occur during specific time periods after transplantation.
Box 6.17
Neutropenic phase (0–30 days)
• Bacterial sepsis
• Opportunistic fungal pneumonia
– Invasive aspergillosis
– Disseminated candidiasis
• Pulmonary edema (engraftment syndrome)
• Diffuse alveolar hemorrhage
• Pulmonary drug toxicity
Early phase (30–100 days)
• Viral pneumonia
– CMV
– Community-acquired respiratory virus
• Idiopathic pneumonia syndrome
• Pulmonary cytolytic thrombi
• Pulmonary venoocclusive disease
Late phase (>100 days)
• Chronic graft-versus-host disease
– Bronchiolitis obliterans syndrome (BOS)
– Organizing pneumonia
• Malignancy
– Posttransplant lymphoproliferative disorder
– Nonmelanoma skin malignancy
• Infection
Neutropenic phase complications (0–30 days posttransplant)
Patients are usually profoundly neutropenic and immunosuppressed in the first month after myeloablative HSCT. Complications that occur in this period may be either infectious or noninfectious in origin. The most common infections are caused by bacterial, Aspergillus, or Candida species. Common noninfectious complications include pulmonary edema, hemorrhage, and drug toxicity. 379,384,386.387. and 388. As noted above, patients receiving autologous transplants are not as profoundly neutropenic, nor as heavily immunosuppressed, and are therefore less susceptible to opportunistic infection during this period. They are, however, at risk for many of the same noninfectious complications as allogeneic transplant recipients. In fact, complications in autologous recipients during this period are often noninfectious in nature. 389
Bacterial sepsis occurs in up to 50% of HSCT recipients, particularly in the first 2 weeks before engraftment takes place. Gram-negative organisms predominate, presumably due to seeding from the gastrointestinal tract. Gram-positive bacteremia is becoming more common, likely due to the use of central venous catheters. The common presence of oral and tracheobronchial ulceration is an additional predisposing factor to bacterial infection in this population. Presumably because of the routine use of prophylactic antibiotics, radiographically evident bacterial pneumonia is uncommon in this period. When it occurs, however, radiographic manifestations are nonspecific and similar to those seen in other groups of immunocompromised patients. Pneumonia initially manifests as focal or multifocal consolidation; rapid progression to more diffuse opacification may occur.
Candida and Aspergillus infections are the most common fungal infections in this period. 388,390,391Candida uncommonly causes isolated pneumonia. More commonly, it causes gastrointestinal or genitourinary infection or results in systemic dissemination. Conversely, Aspergillus has a predilection for the respiratory tract, including the paranasal sinuses. Aspergillosis becomes particularly problematic in recipients of myeloablative allogeneic transplants who have prolonged neutropenia. 388 It is less common in recipients of autologous or nonmyeloablative transplants who have less profound neutropenia. 389 The imaging features of invasive aspergillosis have been previously discussed. Both airway-invasive and angioinvasive forms of the disease are encountered during this period.
Viral infections are uncommon causes of pulmonary disease in the neutropenic phase. Some viruses, however, may cause pneumonia either as a direct pathogen or by indirectly potentiating bacterial infection in the first few weeks after HSCT. Herpes simplex virus primarily causes oropharyngeal ulceration in this setting. Such infection is a risk factor both for bacterial sepsis and for herpes simplex pneumonia, which may result from contiguous spread from the airways. Pneumonia acquired in this fashion manifests with focal or multifocal bronchocentric opacities (Fig. 6.73). Conversely, pneumonia acquired by viremia manifests with more diffuse pulmonary opacities. CT can show multifocal consolidation, ground-glass opacities, and centrilobular nodules. 392 Herpes simplex pneumonia should always be considered in HSCT recipients with evidence of extrapulmonary herpetic infection . Respiratory viruses such as respiratory syncytial virus, adenovirus, influenza, and parainfluenza have also been implicated in cases of early posttransplant pneumonias.393.394.395. and 396.
Pulmonary edema, due to cardiac failure, fluid overload, or capillary leak, is a frequent cause of symptoms and radiographic abnormalities in the immediate posttransplant period. 386 The so-called engraftment syndrome occurs in this early period and is characterized by fever, skin rash, and capillary leak. 382,383,397,398 It occurs in one-third397 to one-half398 of patients. Autologous HSCT recipients are more commonly affected than allogeneic HSCT recipients. 387 Alterations in renal and hepatic function are common. Pulmonary edema occurs in about half of affected patients. 397,398 Radiographic findings are nonspecific and include bilateral homogeneous opacities, septal thickening, and pleural fluid. The syndrome is usually self-limiting and does not progress to ARDS. 397 Sepsis and/or preexisting capillary injury from conditioning regimens may potentiate edema, as may profound hypoalbuminemia. Pulmonary venoocclusive disease that results in edema and pulmonary hypertension has also been reported in HSCT recipients. 382,387,399,400
Diffuse alveolar hemorrhage occurs in up to 21% of transplant recipients (allogeneic and autologous), usually within the first few weeks after transplant, and has a high mortality, between 50% and 80%.382.383. and 384.,386,387,401.402. and 403. There is no apparent association between alveolar hemorrhage in this setting and disorders of coagulation. Hemorrhage usually occurs at the time of engraftment and may represent a complication of leukostasis caused by the sudden influx of neutrophils into the lungs. Affected patients present with dyspnea, cough and sometimes fever. Hemoptysis is quite rare. Diagnosis is established by bronchoalveolar lavage that yields progressively more bloody aliquots of fluid. Chest radiographs usually show diffuse consolidation (Fig. 6.74), but approximately one-third show diffuse reticular opacities. 404 CT can show scattered or diffuse ground-glass opacities that may progress to consolidation (Fig. 6.74).405.406. and 407. These findings are frequently indistinguishable from other complications, particularly drug toxicity and opportunistic pneumonia. Furthermore, diffuse pulmonary hemorrhage tends to occur at about the same time as pulmonary edema.
Conditioning regimens prior to HSCT typically involve some degree of total body irradiation and intensive cytotoxic chemotherapy. These regimens may result in pulmonary toxicity, often in the form of diffuse alveolar damage. These effects may be latent and not manifest for some time after transplantation and may potentiate the effects of previously described complications. Drug toxicity should always be considered in the differential diagnosis of pulmonary complications in the first few months after HSCT and may be a dominant cause of complications in autologous transplant recipients. 389 The histopathologic, clinical, and imaging manifestations of drug- and radiation-induced lung disease are discussed in Chapter 9.
Early phase complications (30–100 days posttransplant)
Early phase complications (30–100 days after transplantation) occur during a period of depressed cellular and humoral immunity. The two most important pulmonary complications in this phase are CMV pneumonia and the idiopathic pneumonia syndrome. Acute graft-versus-host disease (GVHD) in allogeneic transplant recipients also occurs in this period. Acute GVHD occurs 20–100 days after transplantation in 25–75% of patients. 408,409 The effects are predominantly extrapulmonary and include exfoliative dermatitis, diarrhea, and liver dysfunction. Pulmonary manifestations are usually minimal. However, acute GVHD may potentiate the effects of more common pulmonary complications such as infection.
CMV is, overall, the most significant opportunistic pathogen in HSCT recipients, in terms of both morbidity and mortality. 410 The clinical manifestations of CMV infection are extremely varied and include retinitis, encephalitis, esophagitis and enterocolitis, hepatitis, and nephritis. Pneumonia is the most serious manifestation of CMV infection with an incidence of 10%–40% following allogeneic transplantation; mortality rates up to 85% in this setting have been reported. 378 Early diagnosis and treatment with antiviral agents and immunoglobulin can halve the mortality. CMV pneumonia is very uncommon after autologous HSCT. 384 Diagnosis of CMV infection is discussed on page 312, as are the imaging manifestations of CMV pneumonia.
Other viral pathogens can cause pneumonia during this period, including herpes simplex virus (Fig. 6.73) and community-acquired respiratory viruses (Figs 6.75 and 6.76).393.394.395. and 396.,411 Respiratory virus infection is more common in allogeneic than in autologous transplant recipients. Mortality in affected patients can be substantial. In one series, 395 mortality associated with direct respiratory syncytial virus (RSV) and influenza A was 17% and 15%, respectively. Others have reported lower mortality rates, however. 394 Adenovirus is an increasingly important viral pathogen in hematopoietic stem cell transplant recipients, particularly in the pediatric population. 412 Adenovirus can cause hepatitis, hemorrhagic cystitis, and pneumonia in affected individuals.412.413.414.415.416. and 417. Reported mortality rates vary from 1% in patients with localized disease417 to 73% in patients with pneumonia. 416 Pediatric patients and patients with GVHD are at greater risk for severe adenoviral infection after HSCT. Chest radiographic findings of respiratory viral infection in HSCT recipients are nonspecific; CT findings include scattered or diffuse areas of ground-glass attenuation or consolidation, small centrilobular nodules, and thickening of bronchovascular bundles (Fig. 6.76). 411,418
Idiopathic pneumonia syndrome (IPS) is one of the most feared early complications of HSCT. 383,388,419.420.421.422. and 423. IPS is defined as acute lung injury that develops in the absence of demonstrable infectious pathogens, leads to respiratory failure, and manifests histopathologically as diffuse alveolar damage; it is thus a diagnosis of exclusion. It usually occurs within the first 100–120 days after transplant. The overall incidence ranges from 5% to 25% of allogeneic HSCT recipients and it is more common in allogeneic than in autologous transplant recipients. 419 Mortality is high (>70%) and survival after diagnosis is typically short (<2 weeks) despite aggressive management. Diffuse alveolar hemorrhage and IPS are now the major causes of early mortality after HSCT. 423,424 Although the precise pathogenesis of IPS is still unknown, research suggests that donor T lymphocytes and production of inflammatory cytokines play key roles. 419 Evidence also suggests that the conditioning regimen (drug- and radiation-induced lung injury), number of HLA mismatches, and presence of acute GVHD correlate with development of IPS. 382,383,388,419.420. and 421. Affected patients present with progressive dyspnea, cough, and fever. Chest radiographs show scattered or diffuse opacities that may progress to diffuse consolidation (Fig. 6.77). CT shows ground-glass opacities in early stages; later stages may show more consolidation as well as linear opacities with evidence of architectural distortion.
Pulmonary cytolytic thrombi (PCT) are a very rare early phase complication of allogeneic HSCT. 387 Median time to diagnosis is 2 months after transplantation and is strongly correlated with occurrence of acute GVHD. Described almost exclusively in the pediatric population, PCT manifests as multiple small thrombi in small to medium-sized pulmonary arteries and hemorrhagic infarcts; thrombi are primarily composed of monocytes of donor and recipient origin. 425 Affected patients usually present with cough, fever, and dyspnea. Radiographs and CT may show small peripheral opacities or nodules consistent with infarcts. 387,425
Late phase complications (>100 days posttransplant)
The major late phase complications after allogeneic HSCT are chronic GVHD, obliterative bronchiolitis, organizing pneumonia, and posttransplant malignancies including lymphoproliferative disorder. 379,382.383. and 384.,387,388,425.426.427.428.429.430. and 431. Pneumonia, both idiopathic and infectious, remains a fairly common problem in this period as well. 429 Posttransplant lymphoproliferative disorders (PTLDs) are discussed below on pages 367–369.
Chronic GVHD occurs in one-third to one-half of patients surviving more than 100 days after allogeneic HSCT. 408,432 GVHD is, as previously noted, not encountered in recipients of autologous HSCT. Clinical manifestations are similar to those of the autoimmune diseases; scleroderma, primary biliary cirrhosis, and the sicca syndrome may result. The precise incidence of pulmonary disease resulting from chronic GVHD is not well known. Ten percent of patients in one series developed late noninfectious pulmonary complications that were thought related to chronic GVHD. 433 Obliterative bronchiolitis and organizing pneumonia are the most recognized clinical, pathologic, and imaging manifestations of chronic GVHD within the lung.382.383. and 384.,387,388,425,427,428,430,431 It is possible that other described complications, including late-onset idiopathic pneumonia and venoocclusive disease, may also be manifestations of chronic GVHD.
Obliterative bronchiolitis affects 2–26% of allogeneic HSCT recipients who survive longer than 3 months; median onset is 1 year after transplantation. 387,425 Risk factors in addition to chronic GVHD may include use of methotrexate, prior episodes of acute GVHD, prior respiratory viral infection, and the intensity of the pretransplant conditioning regimen. 425,427,428 Fibrous scarring and occlusion of small airways histopathologically characterize obliterative bronchiolitis. Affected patients present with progressive dyspnea and airflow obstruction. The clinical and imaging manifestations of obliterative bronchiolitis in allogeneic HSCT recipients are similar to those of obliterative bronchiolitis in lung transplant recipients and are discussed below on page 359. In short, radiographs are typically normal, especially at presentation. Early CT shows mosaic attenuation and air-trapping; later CT shows progressive bronchiectasis (Figs 6.78 and 6.79). Prognosis is fairly poor, with mortality due to respiratory failure or secondary infection reported in up to 100% (mean 61%) of affected patients. Patients with obliterative bronchiolitis secondary to chronic GVHD can also develop a pattern of progressive upper lobe fibrosis that can be complicated by small and recurrent pneumothoraces. 434,435
Organizing pneumonia is less common than obliterative bronchiolitis after HSCT, and is reported in up to 10% of allogeneic transplant recipients. 387,425,427,428 Although the primary risk factor for organizing pneumonia after HSCT is thought to be GVHD, other potential risks include pulmonary drug toxicity and undiagnosed infection. The histopathologic, clinical, and imaging manifestations of organizing pneumonia in HSCT recipients are similar to those of cryptogenic organizing pneumonia and are discussed in Chapter 10




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree