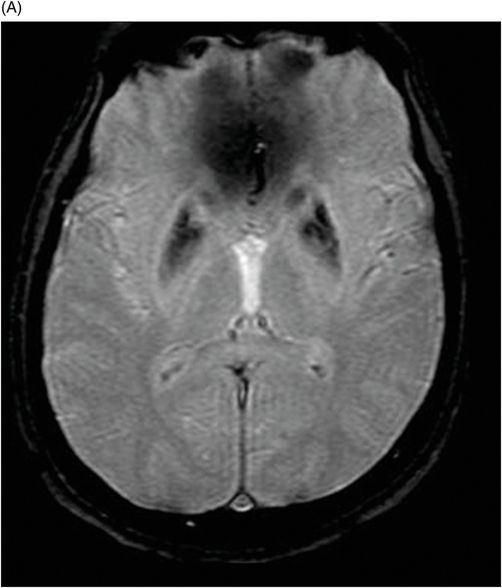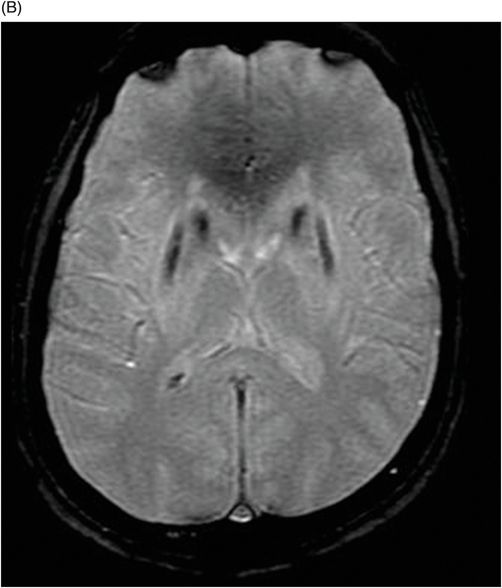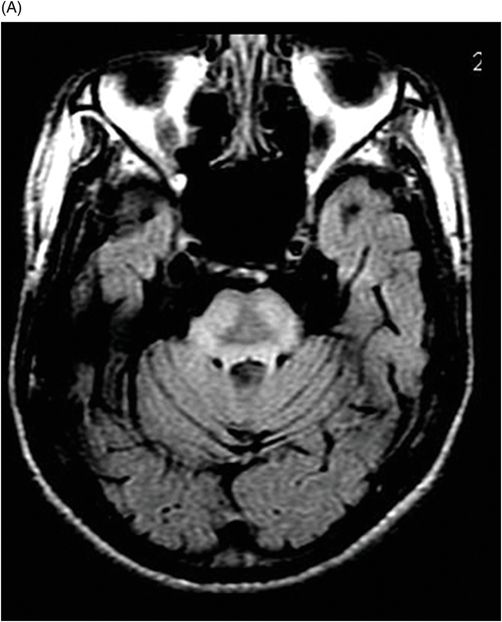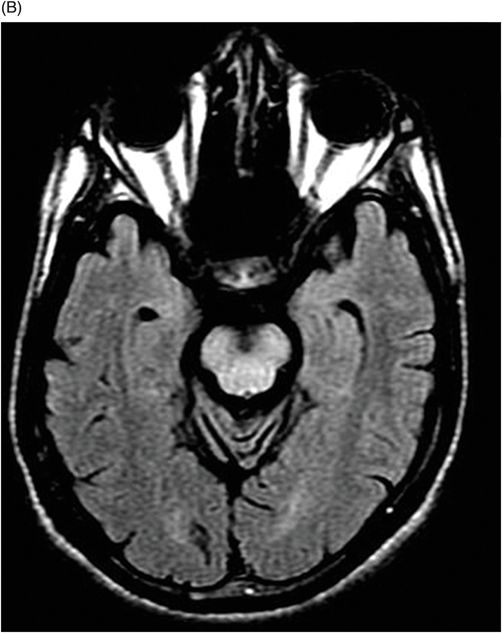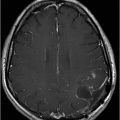
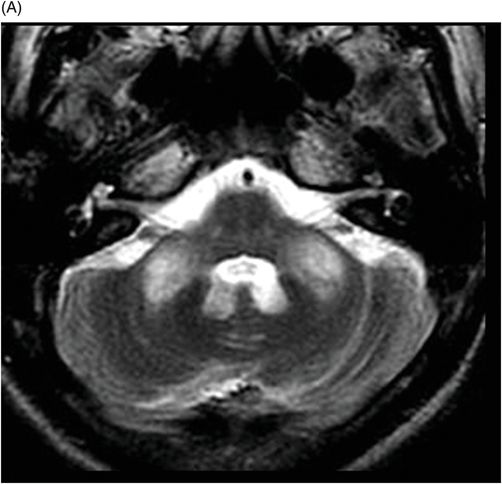
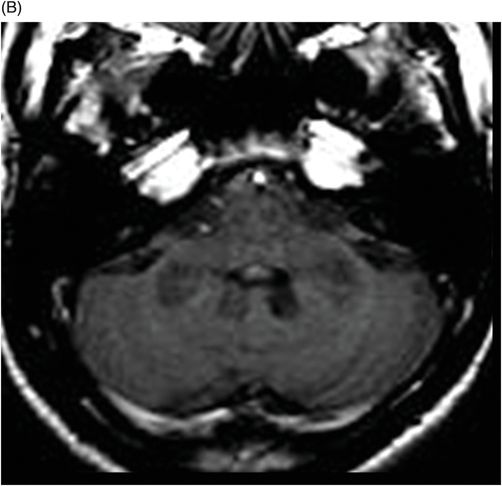
(A) Axial T2WI, and (B) Axial T1-weighted MR postgadolinium images through the level of the middle cerebellar peduncles.
Wilson Disease
Primary Diagnosis
Wilson disease
Differential Diagnoses
Methanol encephalopathy
Leigh disease
Carbon monoxide intoxication
Hepatic encephalopathy
Iron deposition disorders, including pantothenate kinase-associated neurodegeneration 2 (PKAN2) and neuroferritinopathy
Imaging Findings
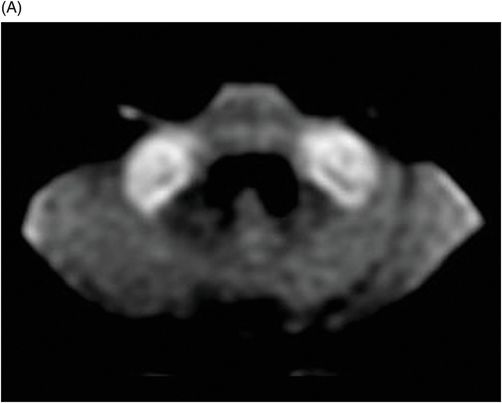
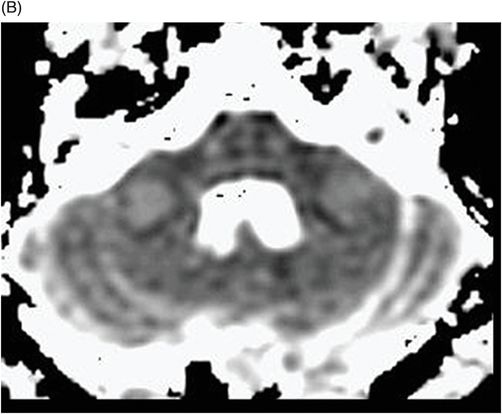
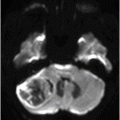
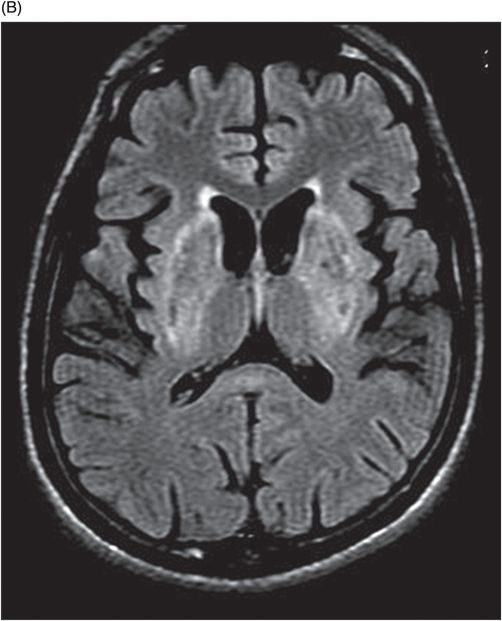
Fig. 75.1 (A–B) Axial FLAIR MR images at the level of the deep nuclei showed hyperintensity in the outer rim of the deep gray matter. Fig. 75.2 (A–B) Axial MPGR-T2*-weighted images demonstrated marked hypointensity in both putamen and caudate nuclei. Fig. 75.3 (A) Axial T2-weighted MR image showed hyperintense areas on both median cerebellar peduncles. (B) Axial T1WI after gadolinium injection showed no enhancement. Fig. 75.4: (A) Axial diffusion and (B) ADC map showed no restriction in the pons or middle cerebellar peduncle lesions. Fig. 75.5: (A–B) Axial FLAIR showed presence of signal abnormalities in the pons and midbrain.
Discussion
Wilson disease (WD) or hepatolenticular degeneration is a rare autosomal recessive disorder of copper metabolism that affects multiple organs, especially the liver and the CNS. A chromosomal 13 mutation in the ATP7B gene causes the dysfunction or absence of the copper intracellular transport protein, ATP7B. As a result, copper deposition occurs in susceptible tissues. The absence of copper in the ceruloplasmin protein produces an unstable molecule, resulting in low serum copper levels. The presence of altered mental status and coronal abnormalities, in combination with distinctive imaging findings, suggests a diagnosis of WD.
Patients with WD usually present in young adulthood (peak age of presentation between 8 and 16 years of age), with dysarthria, dystonia, and tremors that may be followed by rigidity and ataxia. Psychiatric problems may develop later in life. In neurologically symptomatic patients, dysarthria is the most common symptom, followed by tremor, dystonia, seizure, chorea, and psychiatric disturbance. Typically, a Kayser-Fleischer (KF) ring, caused by copper deposition in the cornea, may be seen on slit-lamp ophthalmologic examination.
Potential WD diagnosis should be considered in patients with unexplained liver disease, cirrhosis, liver failure, or other neurologic symptoms suggestive of WD. The presentation of neurologic or psychiatric symptoms concurrently with evidence of liver disease is suggestive of WD. The disease rarely manifests clinically before five years of age, unless there is a secondary problem exacerbating hepatic disease. Hepatic presentations predominate in the first two decades of life, while neurologic or psychiatric symptoms typically appear in the second or third decade of life. A combination of a low serum ceruloplasmin and the presence of KF rings on slit-lamp exam confirm the diagnosis of WD. In individuals without KF rings, a low ceruloplasmin and elevated hepatic copper content (with exclusion of chronic cholestatic disorders) suggests a diagnosis of WD.
The clinical history and the image abnormalities can exclude most of the major differential diagnoses described above. Methanol encephalopathy causes hemorrhagic necrosis of the putamen characterized by marked hypointensity on T2*-weighted MR images and does not usually involve the brainstem like WD. Patients with carbon monoxide intoxication usually have a prior history of suicide attempt or smoke exposure from a fire accident. On neuroimaging there is involvement of both globus pallidi characterized by hyperintensity with an external rim of hypointensity on T2-weighted MR images probably due to hemosiderin deposition, the hemispheric white matter can also be affected, and like methanol encephalopathy, usually there is no involvement of the brainstem.
The symptoms of Leigh disease typically become evident before two years of age and include psychomotor delay or regression, ataxia, ophthalmoplegia, dystonia, abnormal respiratory rhythm, and cranial nerve palsy. The brainstem, periaqueductal gray matter, midbrain, corpus striatum, and thalami are usually affected, characterized by hyperintensity on T2-weighted images, with restricted diffusion in the acute setting.
Chronic hepatic encephalopathy should also be considered in the differential diagnosis. Neuroimaging findings consist of T1-weighted hyperintensity on globus pallidus due to manganese deposition. In the acute setting, there is hyperintensity on T2-weighted images involving the cerebral cortex, preserving the perirolandic region. Wilson disease can cause hepatic disease, making a differential diagnosis difficult without clinical data. The eye-of-the-tiger sign is relatively specific to PKAN2; however, it can also be found in patients with neuroferritinopathy, but is very uncommon in patients with WD.
Despite the ubiquitous presence of toxic copper within the brain, pathologic findings are primarily limited to the basal ganglia, thalamus, and brainstem. These abnormalities include atrophy, spongy softening, cavitation, a neuronal reduction, increased cellularity, and the presence of Opalski cells (an altered glial cell, originated from degenerating astrocytes in WD). These changes presumably result from an increased amount of extracellular copper, which causes oxidative stress, resulting in cell destruction, chronic ischemia, vasculopathy, or demyelination.
Neuroimaging studies may show evidence of diffuse or focal atrophy of the cerebrum, brainstem, and/or cerebellum. Cerebral MRI signal abnormalities are typically seen in deep gray matter sites such as the putamen, caudate nuclei, and thalami (more common and usually bilateral and symmetric), central white matter (usually asymmetric), and other sites such as the pons, medulla, cerebellum, and cerebral cortex. Both pyramidal and extrapyramidal white matter tracts may be involved in WD. These abnormalities are characterized on CT as cerebral hypodense lesions, and may show variable signal on MRI.
TI hypointense-T2 hyperintense lesional imaging is presumably due to gliosis, edema, and variable necrosis (with or without cavitation), and may be hypodense on CT studies as described above. Hypointense lesions on T2 images are either due to the paramagnetic effects of copper deposition, associated iron deposition, or other agents resulting in preferential T2-proton-relaxation enhancement. On T1 sequences, cerebral lesions are hypointense, if there is no significant hepatic disease. In patients with hepatic failure due to WD, T1 hyperintensity is noted in the basal ganglia (see Part V: Cases 66 and 68). Magnetic resonance diffusion may be abnormal in recently developed lesions, characterized by hyperintensity and low ADC values in affected sites. Since excess copper causes cell injury (leading to inflammation and cell death) it is likely that this finding represents cell swelling associated with inflammation, hence the diffusion restriction.
Wilson disease has two specific MR signaling signs. The first is the face of the giant panda sign at the level of the midbrain that corresponds to high signal in the tegmentum, normal signal in the red nuclei and lateral portion of the pars reticulata of the substantia nigra, and hypointensity of the superior colliculus on T2 images. Dorsal pontine signal abnormalities resemble the face of a panda cub. The second sign is known as the bright claustral sign, and correlates with focal hyperintense signal of the claustrum on long TR sequences. Although specific, these findings are only visible in a minority of patients.
In WD, there is a significant correlation between clinical course and follow-up MR imaging, thus MR imaging studies may be useful for documenting the treatment effect. Most WD patients without neurologic symptoms have normal MR scans; in contrast, most patients with neurologic symptoms have abnormal imaging studies. Clinical findings are correlative with lesion location: 1) patients with bradykinesia and dystonia may have putaminal lesions, 2) dysarthria correlates with both caudate and putaminal lesions, and 3) distractibility of gaze fixation correlates with frontal lobe involvement. Finally, localization and severity of brainstem-evoked potential abnormalities correspond closely to MRI abnormalities of the midbrain and pons.
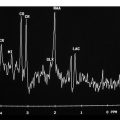
Magnetic resonance images may demonstrate a significant decrease in the NAA/Cr ratio and an increase in the mI/Cr ratio in basal ganglia. The findings could possibly be assigned to neuronal loss (in the three studied areas), to gliosis, and to iron and/or copper deposition in the basal ganglia.
Treatments for WD include administration of zinc, which acts by blocking intestinal copper absorption, and chelating agents, which remove copper from the body, increasing urinary copper excretion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree