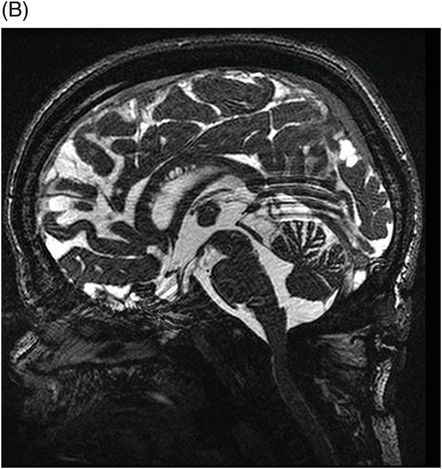
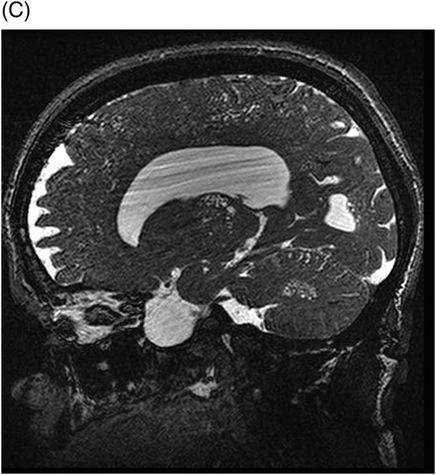
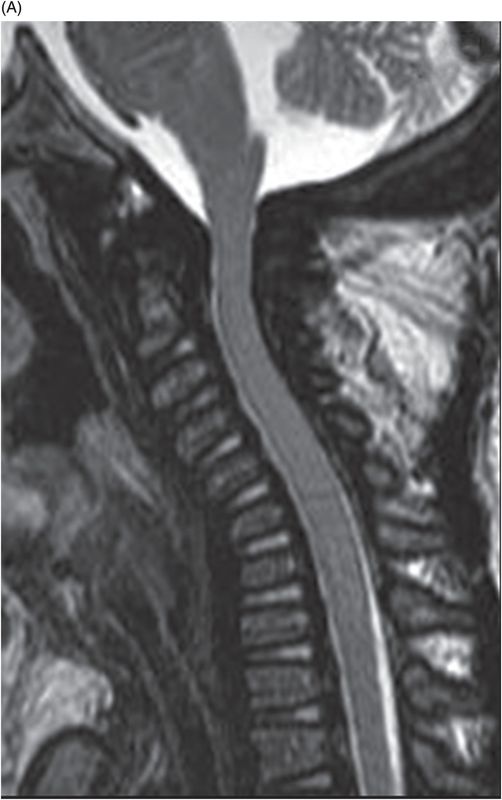
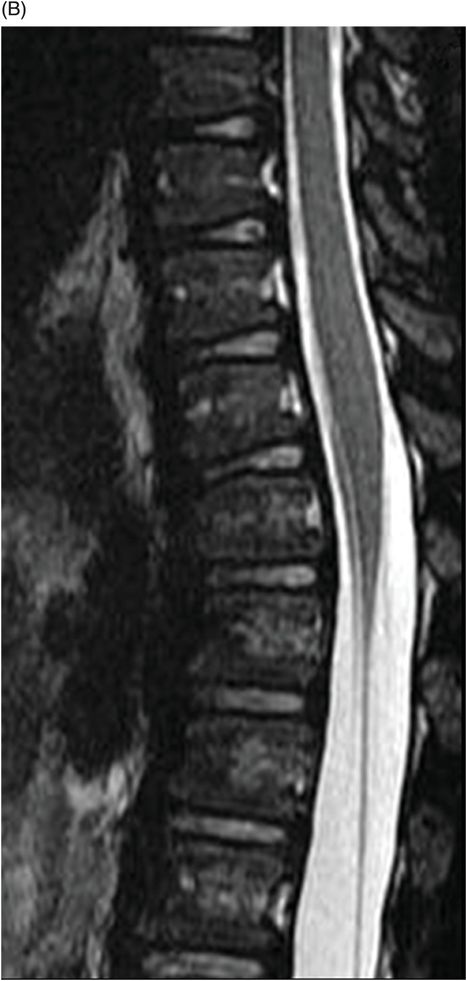
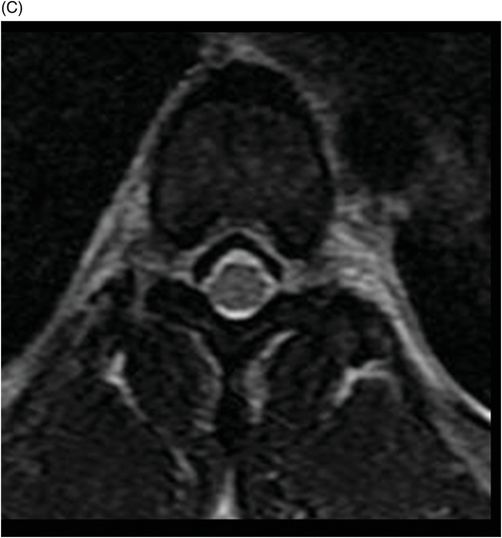
(A–B) Sagittal T2WI and (C) Axial T2WI of the spine through the midline and at the level of dorsal vertebral body.
Mucopolysaccharidosis – MPS Type II (Hunter Syndrome)
Primary Diagnosis
Mucopolysaccharidosis – MPS type II (Hunter syndrome)
Differential Diagnoses
Dilated perivascular spaces (normal variant)
Lowe syndrome
Hypomelanosis of Ito
Foramen magnum stenosis
Down syndrome
Rheumatoid arthritis associated with atlantoaxial subluxation
Imaging Findings
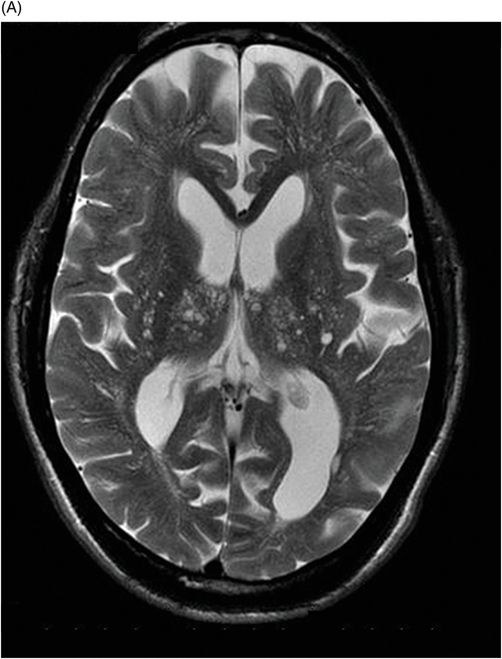
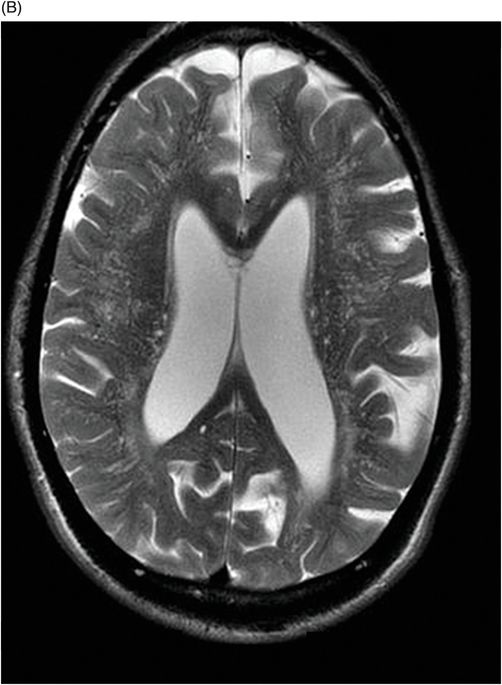
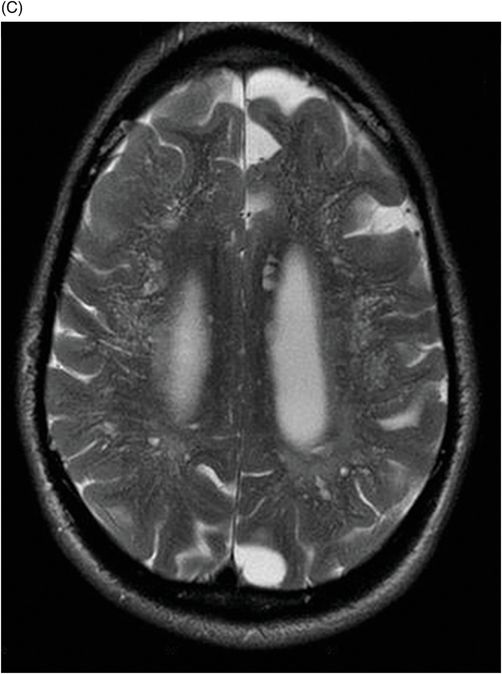
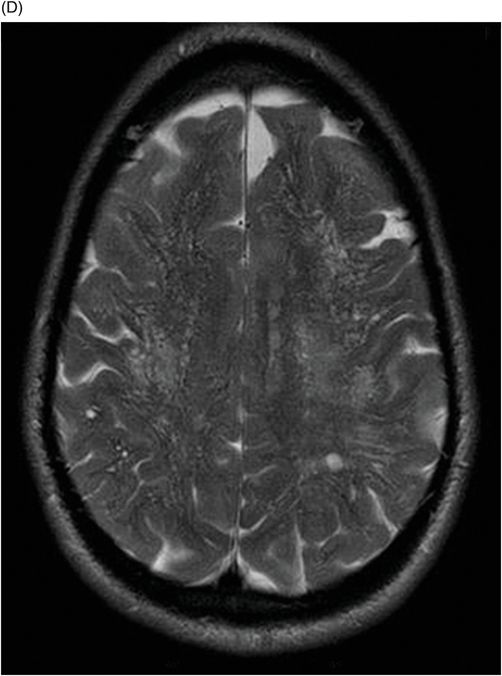
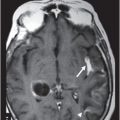
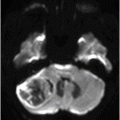
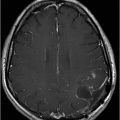
Fig. 82.1: (A) Sagittal T1-weighted and (B–C) Sagittal 3D-CISS MR images of the brain showed prominent perivascular spaces in white matter and deep gray matter. Fig. 82.2: (A–D) Axial T2-weighted MR images showed prominent perivascular spaces in the corpus callosum and deep white and gray matter. Fig. 82.3: (A–B) Sagittal T2-weighted and (C) Axial T2-weighted MR images of the spine showed posterior scalloping and anterior beaking in the vertebral bodies. Foramen magnum and cervical spinal stenosis is evident, due to the thickened dura mater.
Discussion
The MR imaging findings demonstrating presence of perivascular spaces (white matter lesions) and spinal stenosis, in conjunction with the clinical features seen in this patient strongly suggest a diagnosis of mucopolysaccharidosis (MPS). Mucopolysaccharidosis is a heterogeneous group of lysosomal storage disorders caused by an inherited enzyme deficiency. It results in an abnormal accumulation of glycosaminoglycans (GAGs) causing widespread cellular dysfunction and physiologic abnormalities in multiple cell types. Depending on the age of onset, clinical disease features, and associated enzyme deficiency, MPS is categorized into seven broad groups: I (which has three subtypes), II, III, IV, VI, VII, and IX. With the exception of MPS type II (Hunter syndrome), an X-linked disorder, all MPS subtypes are autosomal recessive. It is a multi-organ system disease that primarily involves the skeletal, circulatory, digestive, respiratory, and nervous systems. Depending on the subtype and quantity of residual functioning enzyme, the severity of MPS phenotypic expression varies, as does the impact on connective tissue.
Central nervous system involvement varies widely among MPS groups and individually within each group and subtype. Magnetic resonance spectroscopy may show an abnormal peak at 3.3 to 4.4 ppm, higher than myoinositol, which may represent accumulated GAGs. The most commonly demonstrated CNS imaging abnormality is the appearance of dilated perivascular spaces and white matter lesions/abnormalities. Other imaging abnormalities include brain and optic nerve atrophy, focal or diffuse gray matter abnormalities, cervical cord compression, and hydrocephalus. Profound dilatation of perivascular spaces is primarily indicative of MPS type I and II, although it may be seen in other MPS groups. Dilated perivascular spaces can be seen anywhere in the brain including the corpus callosum (most profoundly in the periventricular white matter). Hydrocephalus may be due to altered CSF absorption from the dural deposition of MPS or increased venous pressure. The development of hydrocephalus in early childhood may cause macrocephaly. Narrowing of the foramen magnum and upper cervical area is a known manifestation of many MPS groups and is usually due to atlantoaxial instability.
Prominent perivascular spaces can be seen in numerous pathologic conditions (such as Lowe syndrome and hypomelanosis of Ito) but may be accidentally found in healthy patients as well. Diagnostic confirmation of MPS can be made in the presence of clinical findings, demonstrated spinal abnormalities, the presence of prominent perivascular spaces, and the thickening of the dura mater, which appears dark on T2-weighted images because of the mucopolysaccharide deposits.
Since MPS adversely affects connective tissue, a vast number of skeletal deformities (dysostosis multiplex) are common, such as anomalies of vertebral shape (platyspondylia and bullet-shaped vertebrae), thoracolumbar kyphosis, odontoid hypoplasia, and atlantoaxial instability, as well as cardiovascular and respiratory symptoms, depending on the group/subtype. Evaluation of spine in the setting of MPS is important to rule out spinal cord compression secondary to spinal involvement that is most common in MPS IV and MPS IS (formerly MPS V). Spinal cord compression in patients with MPS is most commonly at the level of the foramen magnum/upper cervical region.
The definitive diagnosis of MPS can be confirmed by analyzing blood to identify specific enzyme deficiencies. Therapeutic interventions in MPS target correction of enzyme activity through bone marrow, hematopoietic cell, and umbilical cord blood transplantation, as well as enzyme-replacement therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree