with the imaging findings. False-positive and false-negative results are encountered in the evaluation of the acute abdomen. An imaging finding not commensurate with clinical presentation, physical exam, or laboratory studies must be viewed with some suspicion. Knowledge of detailed patient medical data facilitates the radiologist selecting the appropriate imaging modality and improves interpretation and detection of subtle diagnostic findings.
TABLE 14.1 Summary of American College of Radiology Appropriateness Criteria for Selected Gastrointestinal, Genitourinary, and Cardiovascular Conditions | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This series of radiographs comprise the standard abdominal series.
TABLE 14.2 Recommended CT Techniques for Routine Abdomen and Pelvis Imaging | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||
and a pitch of 1.0 to 1.5 is commonly used. Delayed scanning of the kidneys is performed for evaluation of renal excretion with helical axial imaging at 10 mm collimation.
protuberant abdomen, debilitated patients, or patients with little body fat.
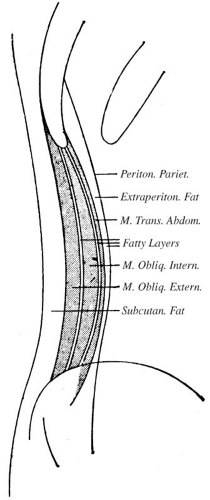
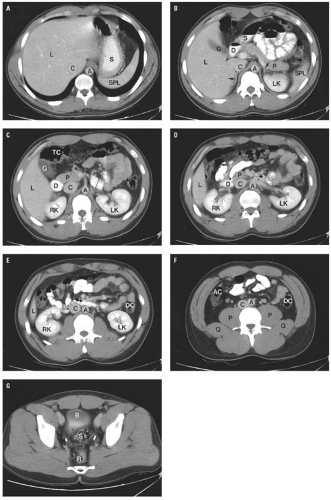
is fat between other structures, the retroperitoneum also contains the great vessels, pancreas, portions of the duodenum and colon, the adrenal glands, kidneys, ureters, and bladder. The extraperitoneal spaces extend from the diaphragm to the floor of the pelvis and wrap around the entire peritoneal cavity. They are divided into the abdominal retroperitoneum, which is the subject of this section, and the pelvic perivesical space, which is described in conjunction with normal anatomy of the pelvis in Chapters 15 and 17.
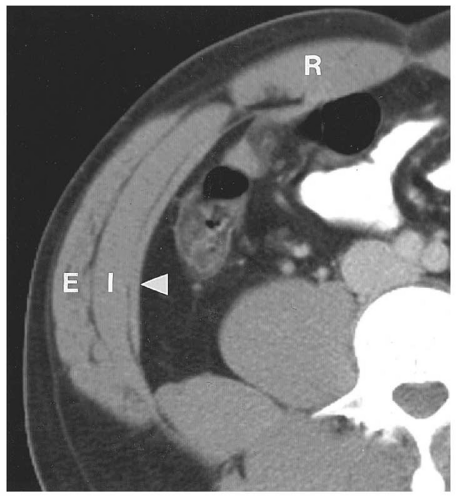
renal fascia and contains only fat. This space becomes important when inflammatory processes track into it.
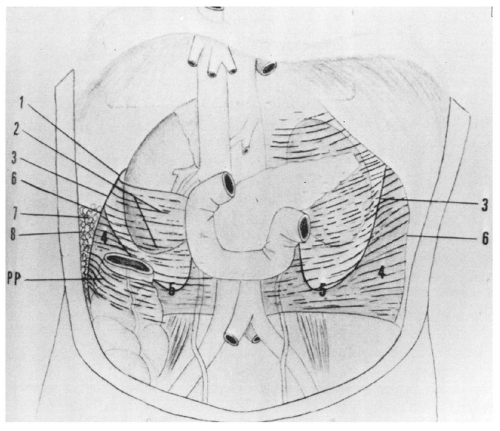
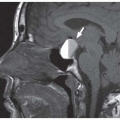
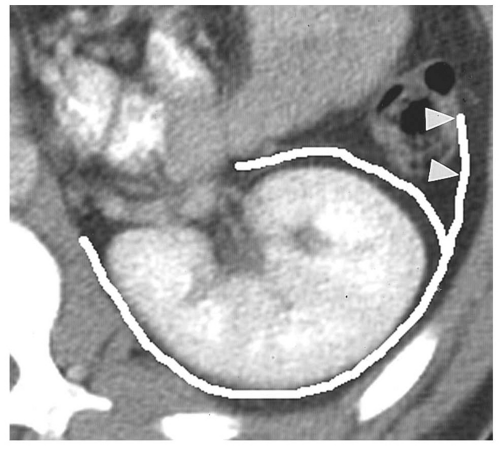 Figure 14.7. Perirenal space. The heavy white line outlines the renal fascia, which merges with the lateroconal fascia extending anteriorly to the descending colon (arrowheads). |
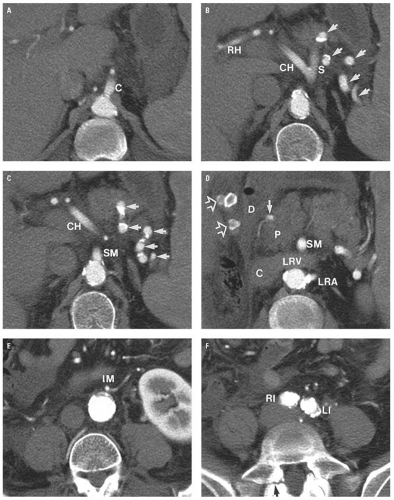
artery arises from the anterior surface of the aorta (Fig. 14.9C). One centimeter below this, the renal arteries arise from the lateral aspects of the aorta (Fig. 14.9D). Just above the aortic bifurcation, the small inferior mesenteric artery arises from the anterior wall of the aorta (Fig. 14.9E).
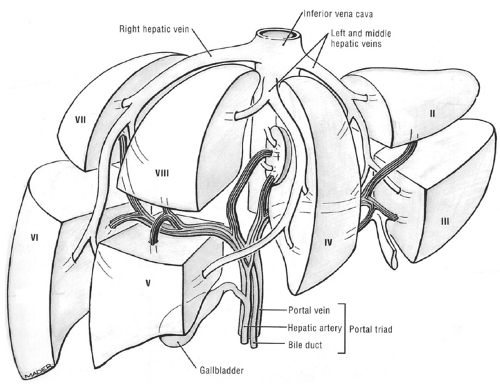
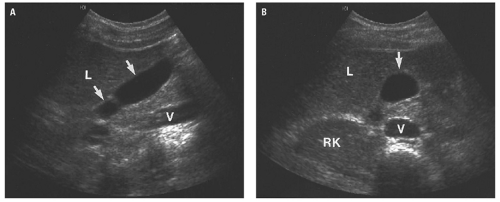
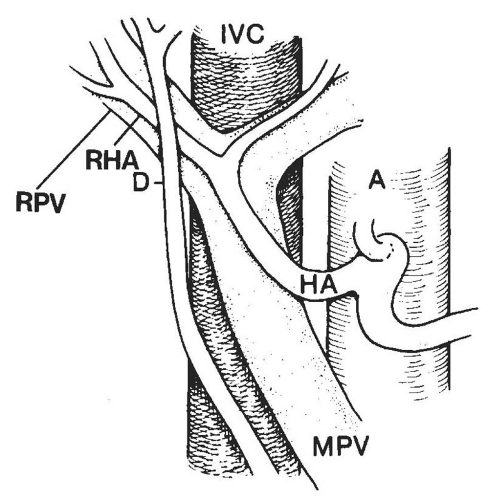
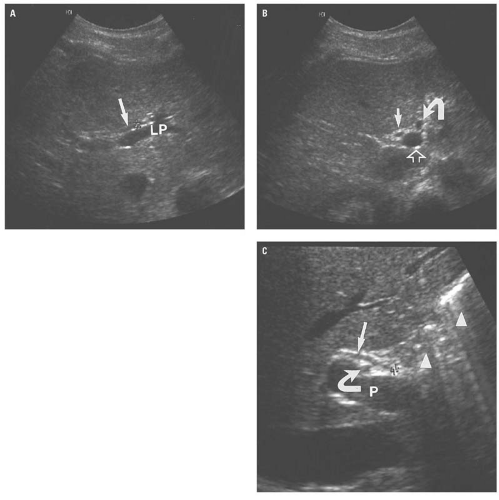
(Fig. 14.13A). Caudally, the common hepatic duct (CHD) is seen anterolateral to the main portal vein. The hepatic artery lies anteromedial to the main portal vein. In cross section, both the CHD and hepatic artery appear as small, round structures, anterior to the portal vein. On the transverse view, the appearance of the CHD, hepatic artery, and portal vein has been likened to Mickey Mouse (Fig. 14.13B) and provides a frame of reference for longitudinal imaging of the CHD and common bile duct. A long segment of the bile duct may be demonstrated longitudinally, anterior to the portal vein (Fig. 14.13C). A portion of the hepatic artery is often seen in cross section between the bile duct and the portal vein. The size of the CHD varies with age and increases slightly after cholecystectomy. Generally, 6 mm is taken as the upper limit of the normal diameter of the CHD in adults. In adults older than 75 years, 10 mm is the upper limit of normal, whereas in children, 3 mm is the upper limit of normal. Distally, the common bile duct shows even greater variability in caliber and may be up to 3 mm larger than the CHD.
TABLE 14.3 Couinaud-Bismuth Segments and Corresponding Liver Anatomy | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||
contrast material. CT usually demonstrates the common bile duct passing through the head of the pancreas.
in the caliber of the ureter through its course from the renal pelvis to the bladder.
of the passage of liquid contrast material. The appearance of the small bowel is variable and depends on the degree of distention by gas or contrast material. The normal, nondistended small bowel wall is thick, but when completely distended by gas or contrast material, the wall measures no more than 3 mm in thickness. The colon is usually not well distended, so wall thickness is difficult to assess; however, when distended, the colon wall is typically less than 3 mm thick.
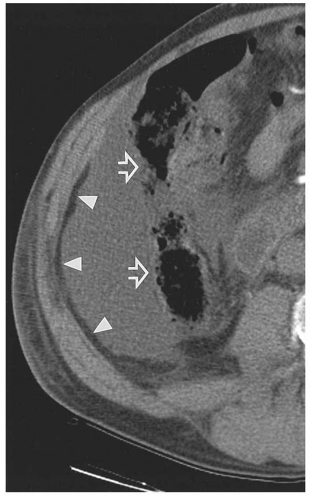
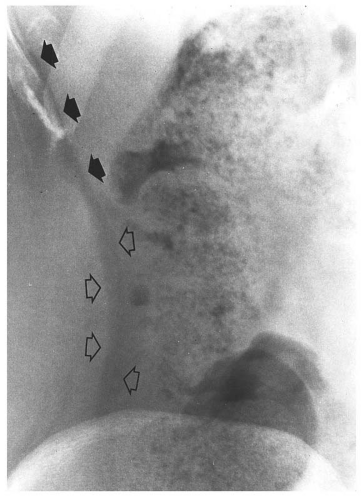
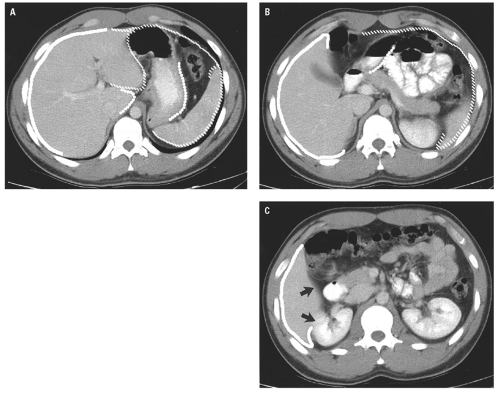
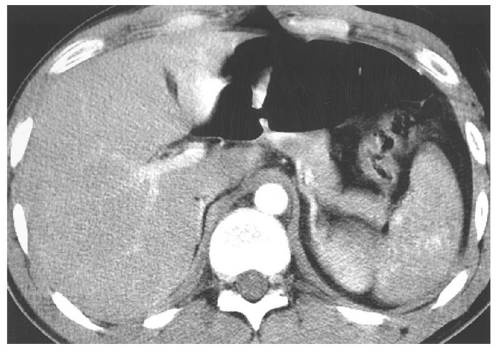
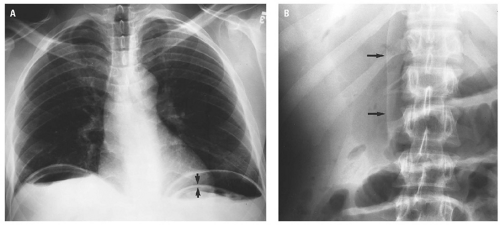
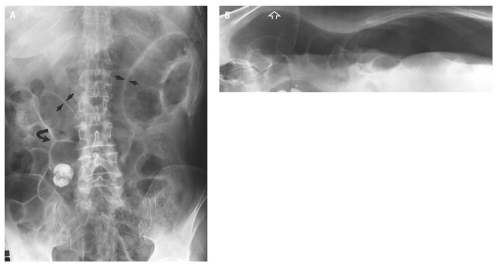
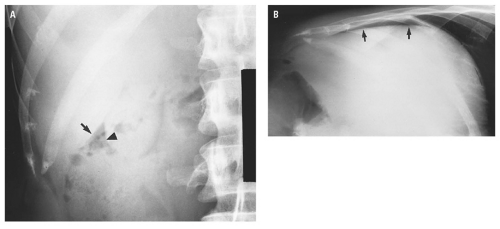
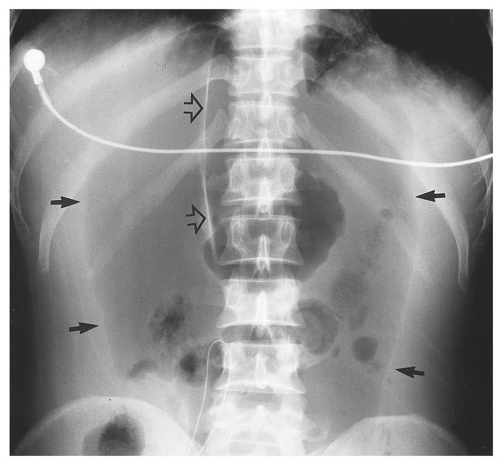
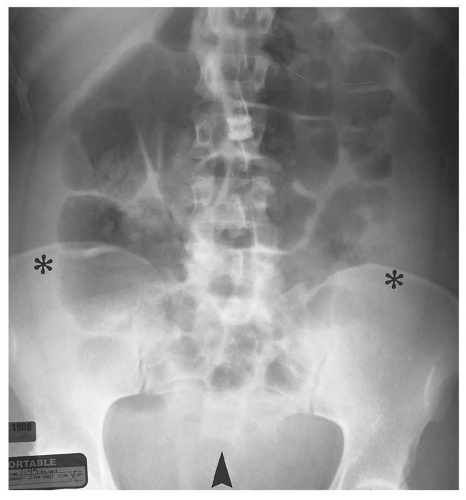
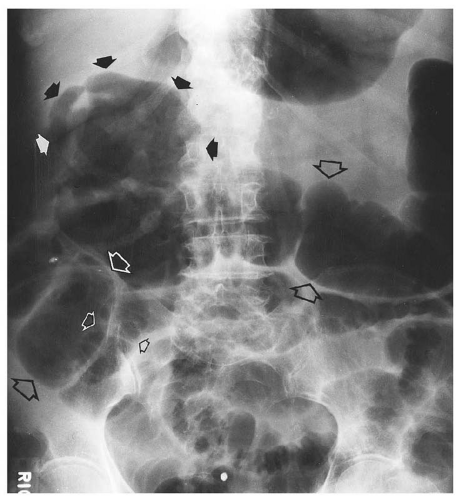
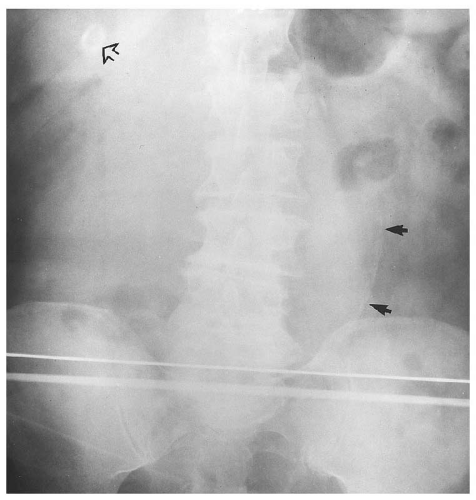
medial half of the right upper quadrant of the abdomen below the hemidiaphragm is the most frequent juxtahepatic pattern of pneumoperitoneum (Fig. 14.17B). Other right upper quadrant signs include visualization of the falciform ligament as a thin linear density over the liver (Figs. 14.17B and 14.20); air within the hepatorenal recess (Morrison pouch) (Fig. 14.19A); extraluminal air outlining the serosal surface of bowel loops (Rigler sign) (Fig. 14.18); and a large, lucent, oval air collection in the midabdomen (football sign) (Fig. 14.20).
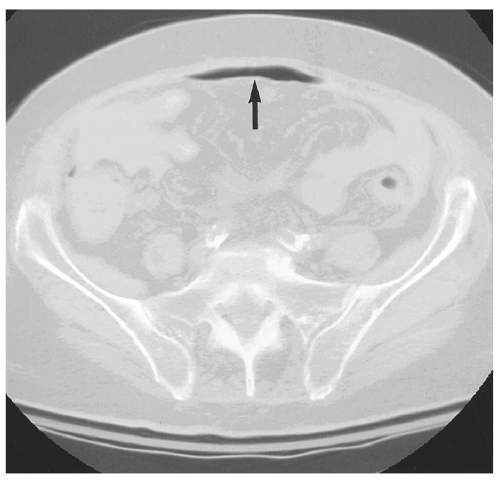
pouch. Findings can be subtle, with pneumoperitoneum manifesting as only a few trapped air bubbles in the mesentery. Wide window settings of more than 750 HU can help detect subtle air collections.
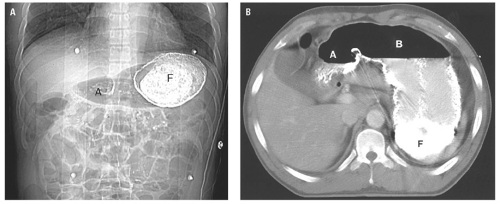
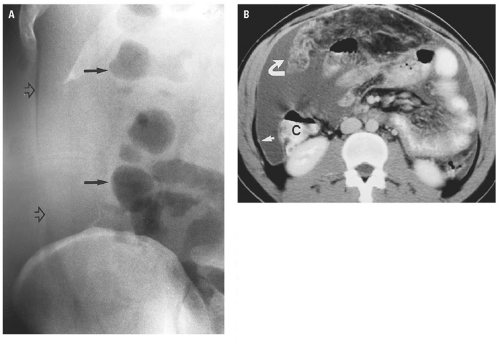
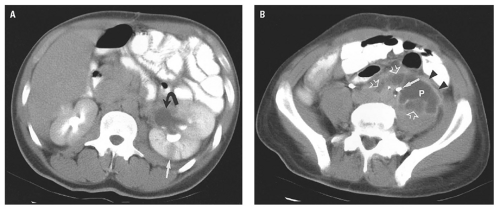
(Fig. 14.25). Extraperitoneal abscess of the psoas muscles conforms to the shape of the muscle but may cause mass effect on adjacent structures (Fig. 14.26). Abscesses contain fluid with low attenuation near that of water. One-third contain small air bubbles or air-fluid levels. An enhancing rim of granulation tissue is characteristic, particularly as the abscess matures, but is present in only a minority of cases. Thickening of fascial planes and soft tissue strands infiltrating fat adjacent to the abscess are signs of inflammation that help distinguish an abscess from sterile fluid collections. However, when the characteristic features of abscess are absent, aspiration may be required to establish the diagnosis.
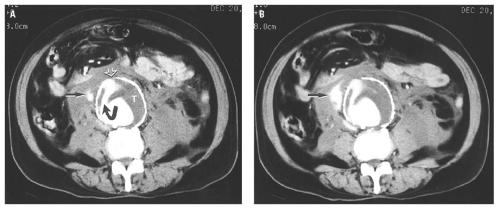
material should be administered to provide additional information about the size and location of intraluminal thrombus and to demonstrate extravasation from a contained rupture (Fig. 14.29). CT is able to show the principal diagnostic sign of a ruptured AAA, a periaortic retroperitoneal hematoma, which may extend into the perirenal or pararenal spaces. In the absence of retroperitoneal hemorrhage, signs of impending rupture may be detected, including “hyperdense crescent” sign, a high-attenuation crescent within the wall of the aorta; focal contour bulge; increased size; or the
demonstration of a focal defect in an otherwise calcified aortic wall (Fig. 14.30).
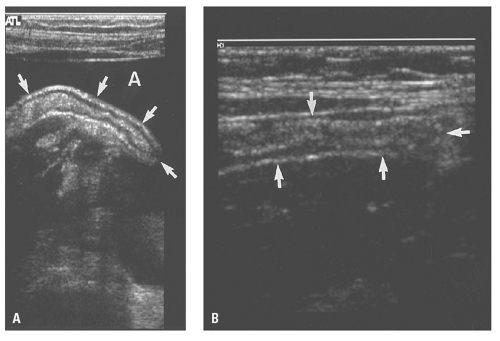
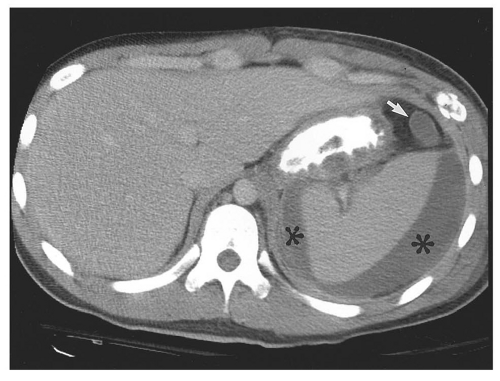
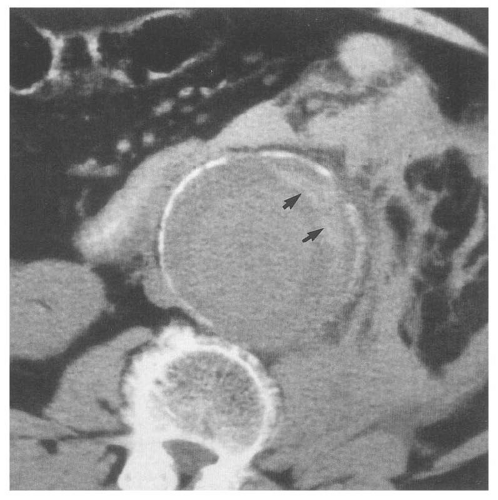
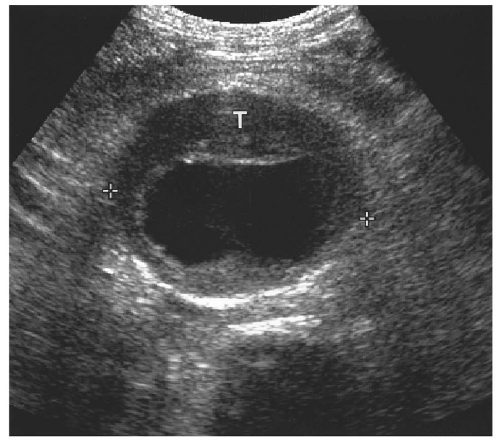 Figure 14.28. Abdominal aortic aneurysm (AAA). Transverse ultrasound image shows 6.8 cm in diameter AAA (electronic cursors). Note the mural thrombus (T) surrounding the lumen. |
arterial extravasation when it is present (Fig. 14.33). An acute hematoma is hyperdense (70 to 90 HU). In hematomas that result from excessive anticoagulation, fluid cell levels and heterogeneity within the hematoma may be seen.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree