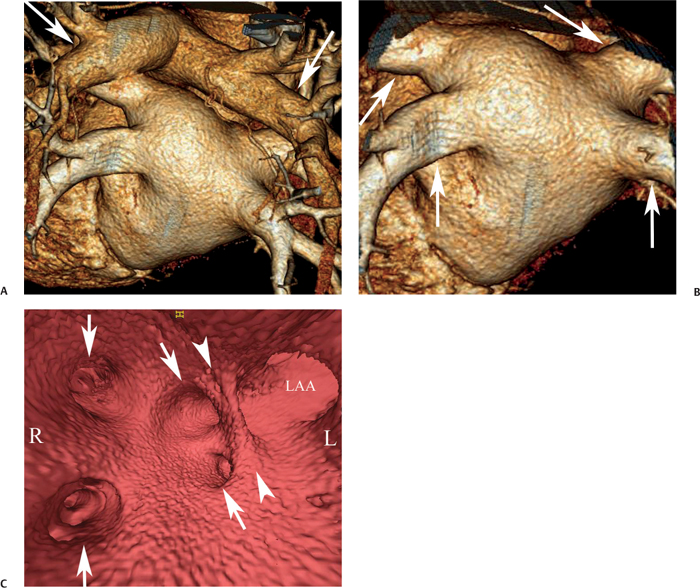
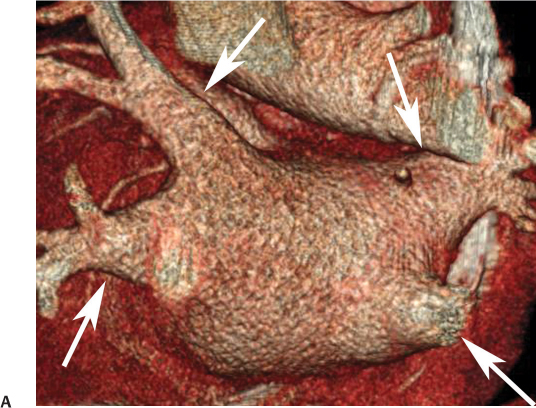
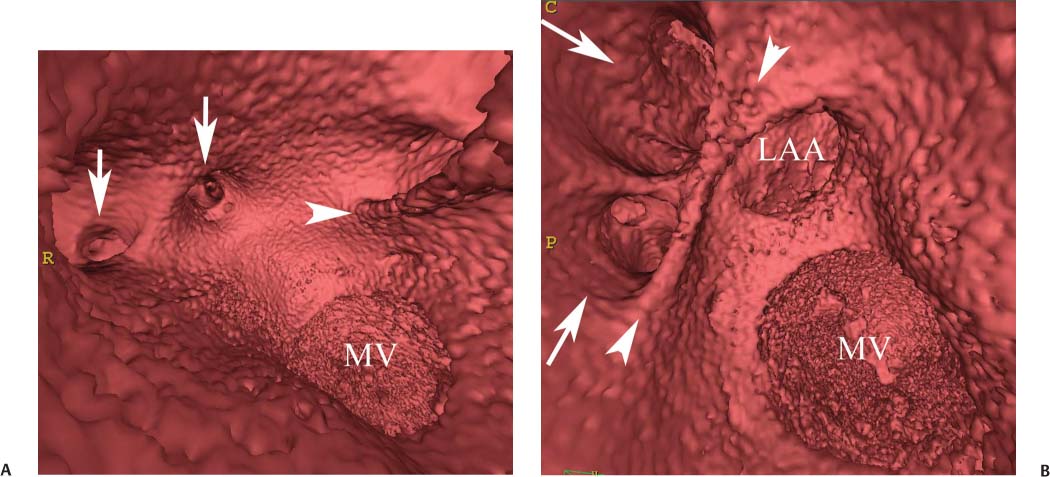
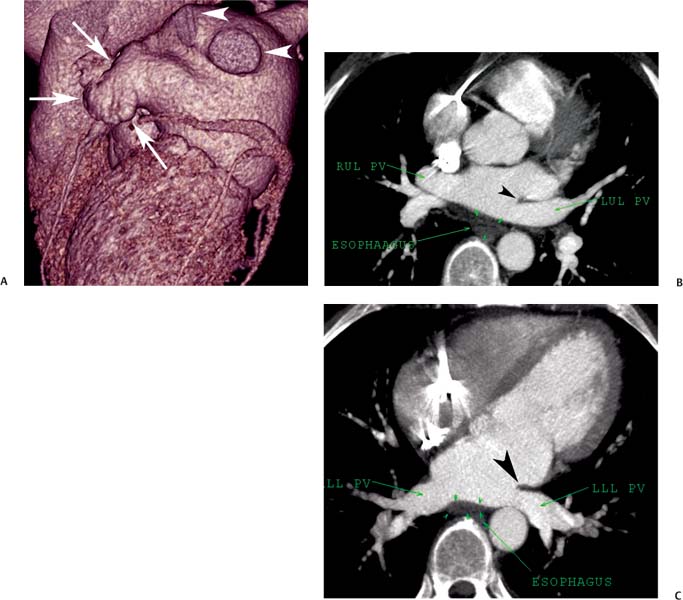
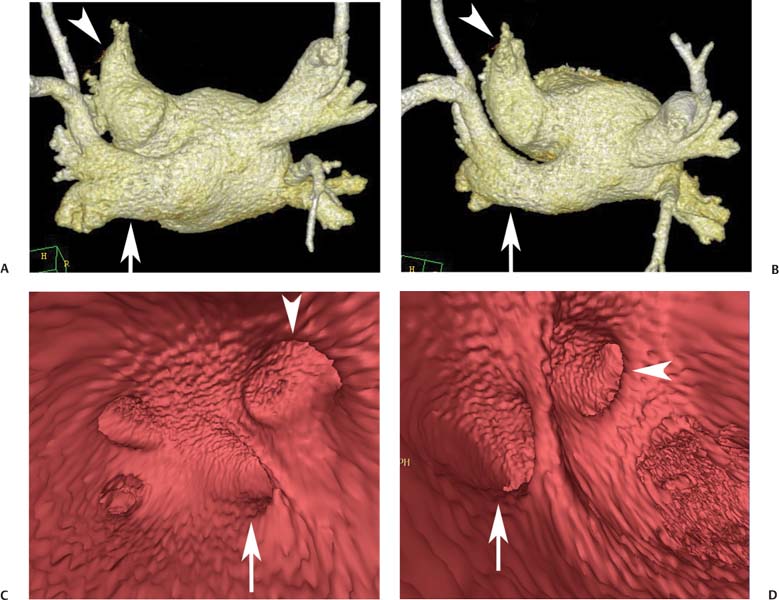
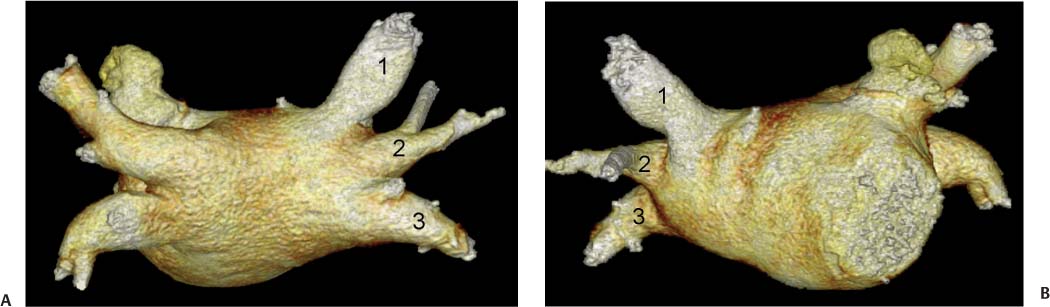
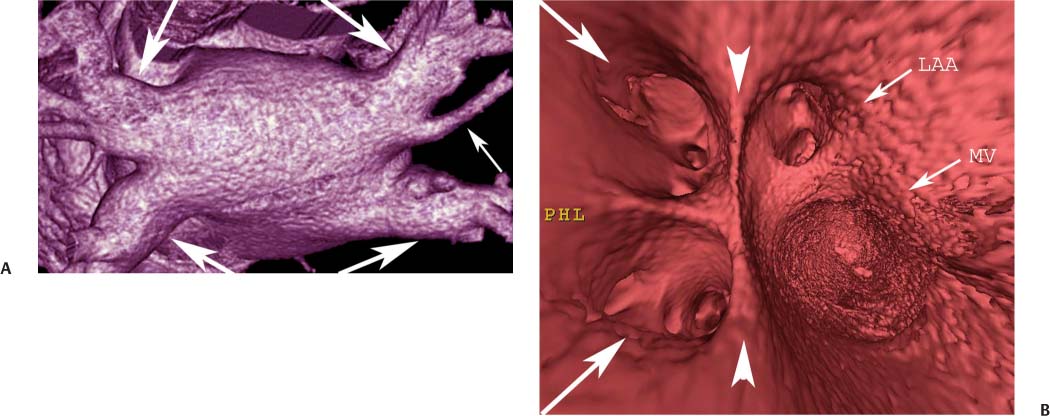
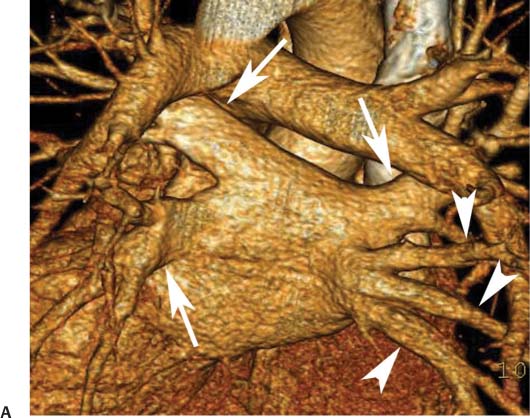
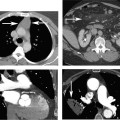
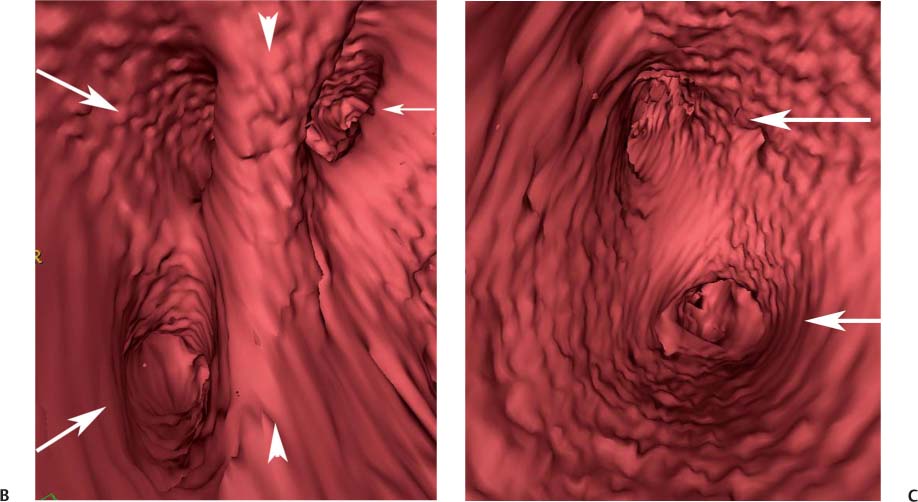
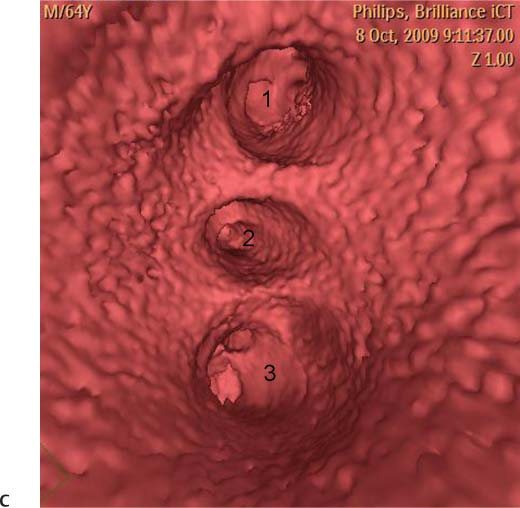
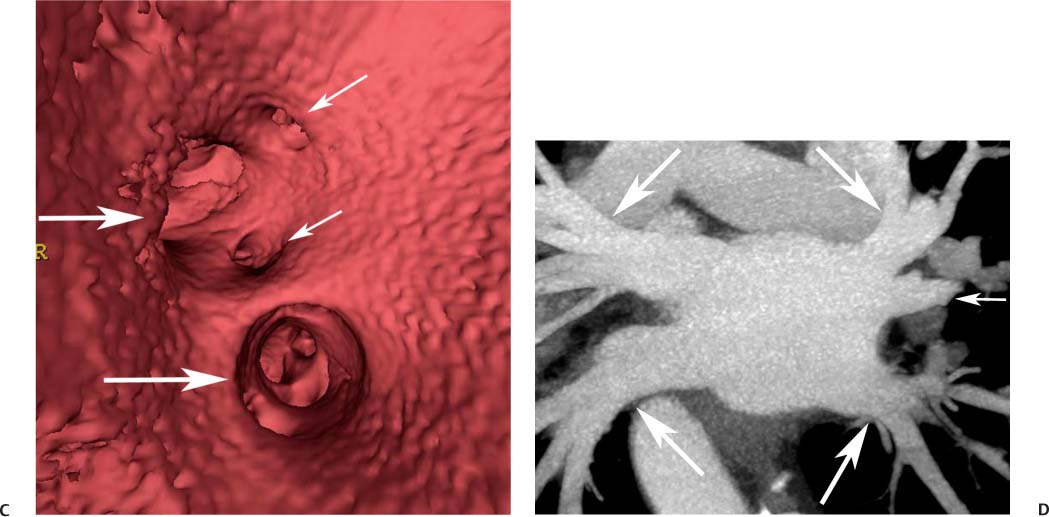
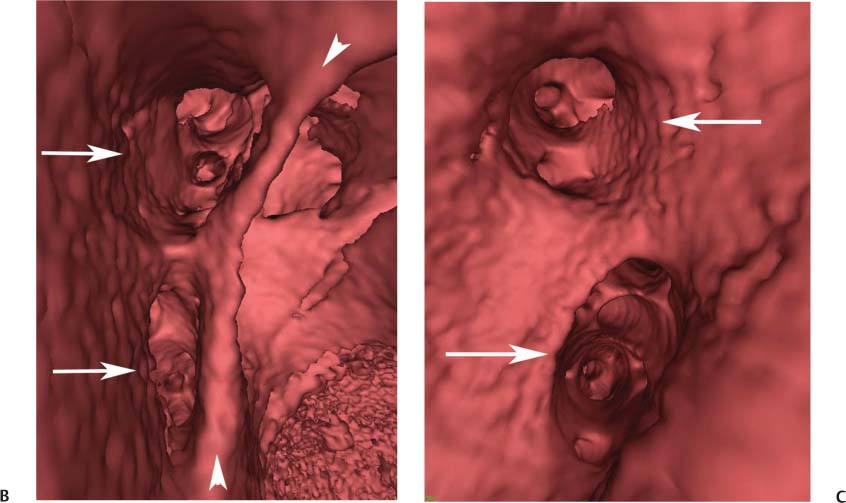
11 Atrial fibrillation (AF) is the most common cardiac rhythm disturbance in humans, with an incidence that increases with age and doubles through many decades of life.1 Because pharmacologic therapy for this arrhythmia is only modestly successful, treatment of AF remains a challenging problem. In the last decade, since the seminal recognition that rapid firing from within the pulmonary veins (PVs) is a common triggering mechanism for AF,2 ablation in the left atrium (LA) has become increasingly common as a treatment strategy. It is now well recognized that the LA musculature extends to variable degrees into the pulmonary veins.3 These extensions of atrial myocardium are the most common sites for rapidly firing triggers that initiate AF. Electrical recordings obtained from circular multi-polar (“lasso”) catheters positioned at the mouth of the PVs reveal sharp electrograms, termed PV potentials. PV potentials are recorded during sinus rhythm and during repetitive firing at the initiation of AF. Although AF may sometimes be provoked by electrical depolarizations from non-PV locations4 (such as the coronary sinus, the ligament of Marshall, the superior vena cava, the crista terminalis, the fossa ovalis), it is clear that most paroxysmal and persistent AF is related to the PVs. Electrical isolation of the PVs has become a central tenet in ablation strategies for AF. Various ablation strategies have been developed to attempt a “cure” for AF. Most of these approaches rely on ablation around the junction of the PVs and the body of the LA, the so-called pulmonary antrum, to achieve electrical disconnection of the PVs from the atrial myocardium. Ablation is most often accomplished with radiofrequency (RF) energy, although alternate energy sources, such as cryoablation, focused ultrasound, and microwave ablation have been investigated. RF energy is delivered around the pulmonary antrum until PV potentials are abolished and electrical disconnection of the PV from the body of the atrium is achieved. Additional RF energy is often delivered in linear patterns (“lines”) to electrically compartmentalize the LA or to create lines of block. Such strategies are anatomically guided and are dependent on a clear understanding of LA anatomy. Not unexpectedly, such extensive ablation strategies in the relatively thin-walled LA are associated with a low, but not insignificant, complication rate. The LA is the most superior and posterior chamber in the heart. The posterior wall of the LA abuts the esophagus. The LA cavity is not amenable to comparison with any standard geometric figure but may be thought of as approximating an oblate spheroid. The mitral annulus is located at the anterior–inferior aspect of the LA chamber. The right and left superior PVs enter the posterior aspect of the “roof” of the LA, whereas the right and left inferior PVs enter at the inferoposterior aspect of the LA, close to the posterior aspect of the mitral annulus (Figs. 11.1 and 11.2). In saggital section, the superior PVs are often more anterior than the inferior PVs, yet the superior PVs are farther from the mitral annulus because of the anteroinferior orientation of the mitral annulus (Fig. 11.3). This anatomic relationship is of clinical importance because ablation strategies often involve creation of a “line of block” by placing contiguous RF lesions from the inferolateral mitral annulus to the left inferior PV to prevent LA flutter.5 On average, the left-sided PVs tend to be slightly larger than the right-sided PVs, and the superior PVs tend to be slightly larger than the inferior PVs. PVs in patients with AF tend to be larger compared with those in control groups without AF. Often the PV ostia are oval rather than circular.6 The LA appendage is a highly trabeculated, “cauliflower-like” protuberance from the upper left side of the LA, extending anteriorly along the left superolateral margin of the LA (Fig. 11.4). The orifice of the LA appendage is generally anterior to the insertion of the superior left PVs, but it may be positioned slightly superior to or slightly inferior to the left superior PV.7 The mouth of the LA appendage is oval and tends to be larger in patients with AF compared with normal hearts. The posterior wall of the LA appendage abuts the anterior walls of the left-sided PVs. The posterior walls of the left-sided PVs often abut the esophagus and descending aorta. The ridge or crest of tissue separating the left PVs from the appendage may be visualized on axial images (Fig. 11.4) or endoluminal images (Figs. 11.1C, 11.2B, and 11.3B). This ridge is sometimes called the “warfarin ridge” because it can be mistaken for a LA clot during echocardiography. The thickness and orientation of this ridge are of great importance to the electrophysiologist during ablation to achieve PV isolation. It is generally not possible to position the ablation catheter on this ridge. Hence ablation is often attempted on the posterior aspect of this structure (ie, the anterior wall of the left PV antrum). Recent studies have demonstrated that pre-procedural CT reconstruction is useful to classify a wide range of variations in morphology of the left atrial appendage.8 Fig. 11.1 Conventional left atrial anatomy (LAA). (A) Surface image demonstrates the posterior wall of the left atrium with associated pulmonary veins. The pulmonary arteries have not been removed (arrows) and do obscure the pulmonary veins. (B) The pulmonary arteries have now been removed, and the four pulmonary veins are clearly identified (arrows). (C) Endoluminal view demonstrates four pulmonary veins (arrows). The ridge that separates the two left-sided pulmonary veins from the left atrial appendage is clearly defined (arrowheads). Fig. 11.2 Conventional left atrial anatomy. (A) Surface image demonstrates four pulmonary veins (arrows). There is a superior and an inferior pulmonary vein on each side. (B) Endoluminal view of the left side of the left atrium demonstrates the left pulmonary veins (large arrows), separated from the left atrial appendage (small arrow) by a ridge (arrowheads). (C) Endoluminal evaluation of the right side of the atrium clearly demonstrates two separate right pulmonary veins (arrows). Fig. 11.3 Relationship of pulmonary veins to mitral annulus. (A) Endoluminal view looking posteriorly–inferiorly from the anterior–superior aspect of the left atrium demonstrates the right-sided pulmonary veins (arrows). The left pulmonary veins are blocked from view by the ridge (arrowhead) that separates these veins from the left atrial appendage. The mitral valve (MV) is located anterior–inferior to this ridge. (B) Endoluminal view of the left side of the left atrium demonstrates the left pulmonary veins (arrows), separated from the left atrial appendage (LAA) by a ridge (arrowheads). The MV is anterior–inferior to the pulmonary veins and closer to the inferior pulmonary veins. Fig. 11.4 The left atrial appendage and relationship of the pulmonary veins (PVs) to the esophagus. (A) Surface view demonstrates the left atrial appendage (arrows), which is anterior to the superior and inferior left PVs (arrowheads). (B) Axial image through the left atrium at the level of the appendage demonstrates that the appendage is anterior to the upper left lobe pulmonary vein and separated from the vein by a thin ridge of tissue (black arrowhead). The esophagus is immediately posterior and contiguous with the left atrium. In this case, the wall of the esophagus is very close to the orifice of the left superior pulmonary vein as it enters the left atrium. (C) At the level of the inferior pulmonary veins, the esophagus is directly posterior to the left atrium. The wall of the esophagus is quite close to the orifice of both PVs as they enter the left atrium. The ridge of tissue that separates the superior pulmonary vein from the left atrial appendage continues inferiorly, and although it is smaller at this level, it is still visualized (black arrowhead). Although most patients have the standard pulmonary venous anatomy described above, a substantial minority of the population demonstrates variation in the number, size, and orientation of PVs (Figs. 11.5, 11.6, 11.7, 11.8, 11.9, 11.10, 11.11 and 11.12). This variability is of clinical importance to the electrophysiologist performing LA ablation because the procedure is anatomically driven. A single antrum for the left-sided pulmonary veins is probably the most commonly encountered variation (Fig. 11.5). In addition, we and others have recently reported additional appendage-like diverticula at varying locations in a significant minority of patients.9 Ablation within these “ectopic” or “supernumary” appendages may be required but may also result in complications (Figs. 11.11, 11.12, 11.13, 11.14, 11.15, and 11.16 and following discussion). To achieve adequate electrical isolation of the PVs and to avoid complications, the electrophysiologist must have precise prior knowledge of these anatomic details in the LA chamber and PVs.10 Fig. 11.5 Single antrum of the left-sided pulmonary veins. (A) Surface image from the back of the left atrium demonstrates the left atrial appendage (arrowhead) is superior to the single pulmonary vein that enters the left side of the left atrium (arrow). (B) Surface image from a cranial position demonstrates the roof of the left atrium with the left atrial appendage anteriorly (arrowhead) and the solitary left pulmonary vein more posteriorly (arrow). (C) Endoluminal view of the posterior wall of the left atrium demonstrates the orifice of the left atrial appendage (arrowhead) superior and anterior to the orifice of the single left pulmonary vein (arrow). These two structures are separated by the warfarin ridge. The two right-sided pulmonary vein orifices are also visible. (D) Close-up endoluminal view again demonstrates the orifices of the left atrial appendage (arrowhead) and left pulmonary vein (arrow) separated by the warfarin ridge. The mitral valve annulus is visible on the bottom right of the image. Fig. 11.6 Accessory right pulmonary vein. (A) Surface image of the back of the left atrium demonstrates three right-sided pulmonary veins (numbered). (B) Surface image in an anteroposterior projection again demonstrates three numbered right-sided pulmonary veins. The left-sided pulmonary veins are obscured by the left atrial appendage. Accessory right pulmonary vein. (C) Endoluminal view along the right side of the left atrium demonstrates the three pulmonary veins (numbered). Fig. 11.7 Small accessory right pulmonary vein. (A) Surface image of the posterior left atrium demonstrates four large pulmonary veins (arrows), as well as a small accessory right middle pulmonary vein (small arrow). (B) Endoluminal view of left side of the left atrium demonstrates the left pulmonary veins (arrows), which are separated from the left atrial appendage (LAA) and mitral valve (MV) annulus by a thin ridge of tissue (arrowheads). Evaluation of the thickness of this ridge is important because it may dictate how the ablation is performed in this area. (C) Endoluminal view on the right side demonstrates two large pulmonary veins emptying into the left atrium (arrows). Although the surface image suggested only one accessory vein, the endoluminal image demonstrates two accessory veins (small arrows), emptying into the left atrium near the insertion of the superior right pulmonary vein. (D) Maximum intensity projection image through the left atrium again demonstrates the four larger pulmonary veins emptying into the left atrium as well as one of the accessory pulmonary veins (small arrow). Fig. 11.8 Compound inferior right pulmonary vein. (A) Surface image of the posterior left atrial wall demonstrates three large pulmonary veins (arrows), representing the left superior and inferior pulmonary veins, as well as the right superior pulmonary vein. The right inferior pulmonary vein is composed of three smaller vessels that empty into a common orifice (arrowheads). Compound inferior right pulmonary vein. (B)
Ablation in the Left Atrium
 Pathophysiology of Atrial Fibrillation
Pathophysiology of Atrial Fibrillation
 Ablation Strategies to “Cure” Atrial Fibrillation
Ablation Strategies to “Cure” Atrial Fibrillation
 “Normal” (Conventional) Left Atrial Anatomy
“Normal” (Conventional) Left Atrial Anatomy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree