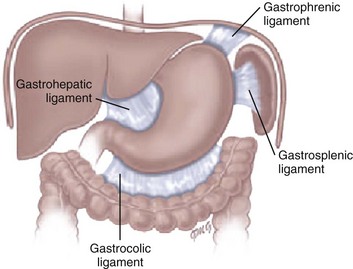
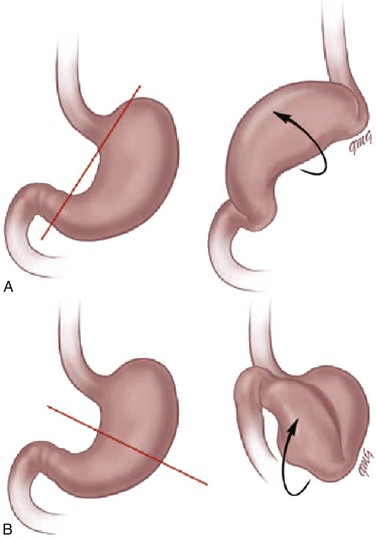
Chapter 102 Etiology: Normally, the stomach is relatively fixed in the peritoneal cavity at the esophagogastric junction, with four additional ligaments: (1) gastrohepatic, (2) gastrosplenic, (3) gastrocolic, and (4) gastrophrenic (Fig. 102-1) Gastric volvulus is defined as an abnormal rotation of the stomach of more than 180 degrees around its long (organoaxial) or short (mesenteroaxial) axes (Fig. 102-2, A and B), causing a closed loop obstruction, with consequences such as incarceration, strangulation, and perforation.1 Predisposing factors for gastric volvulus include congenital or acquired absence of one or more ligaments as isolated abnormalities or conditions such as asplenia and diaphragmatic defects. In organoaxial gastric volvulus, an inversion of the position of the greater and lesser curves of the stomach occurs, with the greater curvature positioned to the right and superior to the lesser curvature. In mesenteroaxial gastric volvulus, the stomach folds on its short axis; this leads to reversal of the relationship between the gastroesophageal junction and the pylorus. Clinically, two primary scenarios exist. The first is the acute fulminant presentation, most often encountered in the mesenteroaxial type, with sudden and persistent vomiting and acute abdominal pain.1 The chronic intermittent presentation is more often associated with the organoaxial type, with less specific symptoms, including recurrent abdominal pain, vomiting, and gastric distension.2 Imaging: Abdominal radiographs in patients with gastric volvulus typically show marked gastric distension. The stomach becomes spherical, with paucity of distal bowel gas, indicating gastric outlet obstruction (Fig. 102-3, A). Other findings include diaphragmatic elevation and the presence of two air-fluid levels in the stomach. Occasionally, the type of gastric volvulus can be inferred by the gastric configuration: a pylorus projecting over the gastric fundus and an unusual nasogastric tube course are suggestive of the mesenteroaxial type, whereas an inversion of the relationship of the greater and lesser curvatures is suggestive of the organoaxial type; mixed types also occur, with combined imaging findings. Figure 102-3 Mesenteroaxial volvulus in a 6-year-old girl presenting with acute unremitting vomiting. Although, in most cases, plain radiographs are highly suggestive of the diagnosis, the upper gastrointestinal (UGI) series remains the diagnostic procedure of choice, demonstrating the type of volvulus and evidence of gastric outlet obstruction. If performed, other imaging modalities such as CT can also be useful in demonstrating the abnormal orientation of the stomach (see Fig. 102-3, B) as well as associated anomalies such as heterotaxy or the presence of pneumatosis.2 Etiology: Spontaneous perforation of the stomach is an uncommon event mainly seen in the neonatal period as a cause of pneumoperitoneum.5 The etiology is unknown, but possibilities include sudden gastric distension with a degree of ischemia attributable to perinatal hypoxia, a more distal bowel obstruction, and congenital focal absence of the muscle of the gastric wall.6–8 Beyond the neonatal period, perforation is rare and usually secondary to trauma (tubes, catheters), surgery (fundoplication), caustic ingestion, or peptic ulcer.1 The most common presenting manifestations of perforation include sudden onset of abdominal distension, ileus, respiratory distress, and, less frequently, cyanosis, fever, vomiting, and bloody stool.9 Imaging: Abdominal radiography is the imaging method of choice when perforation of the gastrointestinal (GI) tract is suspected. As in any other type of GI perforation, abdominal radiographs will typically demonstrate free intraperitoneal air. A reported suggestive sign of gastric perforation is the lack of an air-fluid level in the stomach in a horizontal beam view, and relative paucity of gas in the distal bowel.10 Etiology: Peptic ulcer disease represents ulceration of the gastric or duodenal mucosa resulting from, on the one hand, an imbalance between the mucosal protective mechanisms and, on the other, the aggressive factors of acid and pepsin production, injury, and infection.11 The gel layer, a protective bicarbonate and mucous barrier lining the stomach, is approximately 0.2 to 0.5 mm in thickness and consists of 95% water and 5% mucin glycoprotein. Breaches in this gel layer, secondary to H. pylori or antiinflammatory drugs, result in a continuum of damage to the underlying mucosa, with ulceration occurring when damage extends to the muscular layer. Peptic ulcer disease in children may be primary or secondary (induced by drugs, alcohol, stress, or metabolic disease), with each form having different manifestations and prognostic implications. H. pylori has been recognized as a common human pathogen associated with both inflammatory and malignant conditions of the upper gastrointestinal tract, and affects nearly all children with peptic ulcer disease.12 Primary peptic ulcers are associated with H. pylori infection. In addition to being classified as primary and secondary, peptic ulcers can also be classified according to the site of involvement (gastric or duodenal). Gastric ulcers are mostly seen in neonates and young children, whereas duodenal ulcers are more common after the neonatal period and tend to be secondary to systemic illness or chronic intake of medications such as non-steroidal antiinflammatory agents. Zollinger-Ellison syndrome causes secondary peptic ulcer disease, often with multiple ulcerations caused by increased acid generated by a gastrin-producing tumor (Fig. 102-4).11,13 Figure 102-4 Zollinger-Ellison syndrome secondary to a pancreatic gastrinoma in a 10 year-old-boy. Symptoms of ulcer disease vary with age; infants and young children present with feeding problems and vomiting. In some patients, the first sign of peptic ulcer disease may be upper or lower GI hemorrhage or acute severe abdominal pain due to perforation. Pain can be nocturnal or occur early in the morning. Unlike in adults, the pain is neither precipitated nor relieved by meals or antacid use.11 Imaging: Endoscopy has assumed the primary role in the diagnosis of ulcer disease over the past two decades, while the role of the radiologist has dramatically decreased and is now limited to the incidental case, as UGI contrast studies have been demonstrated to have a high false-negative rate for ulcer detection.14,15 However, these studies, as an initial tool to evaluate the child with abdominal pain and vomiting, may incidentally demonstrate the ulcer. Perforated ulcers may be incidentally identified on CT in the evaluation of a child with acute abdominal pain (Fig. 102-5). Figure 102-5 Perforated duodenal ulcer in 16-year-old boy on nonsteroidal antiinflammatory regimen for previous knee injury and surgery, presenting with acute onset of abdominal pain while in school. Treatment and Follow-up: Current therapy has been proven to be effective and is based on medications that decrease acid production. In the cases of H. pylori infection, a combination therapy, including a histamine-2 blocker or proton pump inhibitor, antibiotics, and, bismuth, may be necessary to eradicate the causative organism and prevent both recurrence and malignant complications.11 Etiology: Hypertrophy of the gastric rugal folds, in association with protein-losing enteropathy, in childhood is labeled Ménétrier disease, or hypertrophic gastropathy of childhood.16 The clinical, pathologic, and etiologic factors of this disease in children differ from those of the adult form. In adults, the disease is chronic and premalignant. In children, the disease is self-limiting, with a peak age of presentation of 5 years. Presentation includes acute vomiting, diarrhea, upper abdominal pain, and anorexia. Peripheral edema is usually present and may be associated with ascites and pleural effusions. Rarely, signs of GI bleeding occur with coexisting ulceration of the gastric rugae. The etiology of the disease remains unknown; however, it has been previously associated with several infectious agents, including cytomegalovirus, H. pylori, mycoplasma, herpes virus, and Giardia lamblia. Imaging: Diagnosis is most commonly made with UGI contrast studies demonstrating thickened gastric mucosal folds in the fundus and body, sparing the antrum and pylorus, with normal appearance of the small bowel.17 Ultrasound has also been successfully used in diagnosis.18 On the CT scan, similar findings of thickened rugal folds in the fundus and body of the stomach can be seen, with sparing of the antrum (Fig. 102-6). Endoscopy confirms the diagnosis. Differential diagnosis includes eosinophilic gastritis, primary gastric lymphoma, gastric carcinoma, inflammatory pseudotumor, gastric varices, Zollinger-Ellison syndrome, lymphangiectasia, and anisakiasis if there is a history of ingestion of raw fish.16 Figure 102-6 Ménétrier disease. Etiology: Chronic granulomatous disease (CGD) of childhood is a hereditary disorder of neutrophil function, which is typically inherited as an X-linked recessive disorder, but three autosomal recessive defects have also been identified. The genetic alteration leads to a defect in activation of the NADPH (nicotinamide adenine dinucleotide phosphate-oxidase) molecule within the phagocyte, preventing the formation of free radical superoxide in the “respiratory burst,” and resulting in survival of catalase-positive organisms within the phagocytes, with chronic inflammatory reaction and granuloma formation.20 In the stomach, narrowing of the gastric antrum is a distinctive manifestation of CGD, occurring in 16% of cases.21 Gastric outlet obstruction occurs in the X-linked recessive form more commonly than in the autosomal recessive form and presents at a mean age of 44 months, usually with severe vomiting.22
Acquired Disorders
Gastric Volvulus
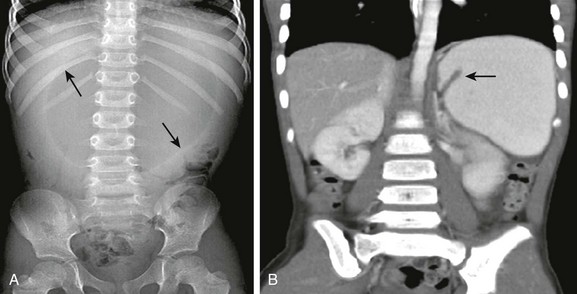
A, Supine abdominal radiograph shows a dilated, spherical gastric bubble (arrows) with paucity of distal bowel gas. B, The coronal image of a contrast-enhanced computed tomography scan shows a reversal of the axis of the distended stomach with the pylorus superiorly located and inverted (arrow).
Spontaneous Gastric Perforation
Peptic Ulcer Disease
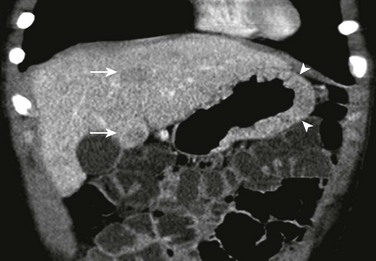
The coronal image of a contrast-enhanced computed tomography shows marked segmental thickening of the gastric fundus and body (arrowheads). Two hypodense lesion in the liver indicate metastases (arrows).
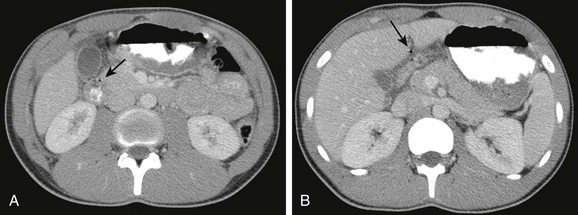
A, A contrast-enhanced computed tomography scan shows marked thickening of the duodenal wall and small amount of free air (arrow). B, The slightly more cephalad image shows fluid about the duodenum and additional free air extending toward the area of the falciform ligament (arrow).
Hypertrophic Gastropathy (Ménétrier Disease)
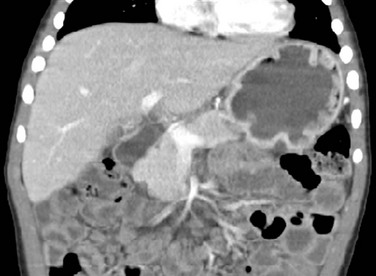
Contrast-enhanced coronal computed tomography reformation shows the typical thickening of the gastric folds in the fundus of the well distended stomach in a 4-year-old boy presenting with 10 days of vomiting with streaks of blood, palpebral edema, and hypoalbuminemia. Ménétrier disease confirmed with endoscopy and gastric biopsy.
Chronic Granulomatous Disease
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree