Fig. 11.1
(a) Pathologic anatomic specimen of a premature born infant. The image shows the large size of the normal adrenal gland in comparison with the kidney with normal renculation. (b) Neonatal adrenal gland (longitudinal section through the right upper abdomen). The image shows the triangular shape of the normal adrenal gland with the outer adult and the inner foetal cortex. (c) Neonatal adrenal gland (transverse section through the right upper abdomen). The inner echogenic foetal cortex is much thicker than the outer adult cortex. IVC inferior vena cava

Fig. 11.2
(a) Neonatal adrenal gland (transverse section through the right upper abdomen). The image shows the large adrenal gland on top of the kidney. GB gall bladder. (b) Neonatal adrenal gland (transverse section through the left upper abdomen). Behind the enlarged spleen the large neonatal adrenal gland is displayed
In the neonatal period, the right adrenal gland can be visualised in 97–100 % and the left in 83–98 % (Figs.11.1b–c and 11.2a, b) (Kangarloo et al. 1986; Scott and Thomas 1990). According to literature, the normal adrenal glands can be visualised in only 71 % older children (Marchal et al. 1986).
The adrenal glands are best visualised by a right or left flank approach (Figs. 11.1 and 11.2). In older children the adrenal glands are best evaluated intercostally. The right adrenal gland is best seen in the anterior or mid-axillary line through the liver as an acoustic window (Fig. 11.1b, c). The left adrenal gland can best be imaged in the posterior or mid-axillary line through the spleen or left kidney (Fig. 11.2b).
11.2 Normal Adrenal Gland
2D Image
The adrenal glands lie within the perirenal fascia.
The right adrenal gland is situated lateral to the diaphragmatic crus, medial to the right lobe of the liver, posterior to the vena cava inferior and anterior superior to the upper pole of the right kidney.
The left adrenal gland is located lateral to the aorta and left diaphragmatic crus, medial to the spleen and posterior to the splenic vessels and pancreatic tail and anteromedial to the upper pole of the left kidney.
Shape: In coronal and longitudinal planes, the adrenal glands have a V or Y shape, whereas in transverse planes they have a linear, V, Y or L configuration (Figs.11.1 and 11.2) (Yeh 1987).
In the case of unilateral renal agenesis or ectopia, the ipsilateral adrenal gland elongates and loses its typical Y or V shape (McGahan et al. 1986). The adrenal gland appears flattened or has a discoid appearance (Fig. 11.3). The elongated and flattened gland retains its normal corticomedullary differentiation (Fig. 11.3).


Fig. 11.3
Flat adrenal gland in a patient with renal agenesis. In renal agenesis the adrenal gland has a linear and no triangular shape
Echotexture: The echotexture of the adrenal glands is age dependent. In the newborn period the medulla is relatively thin and hyperechoic. It is surrounded by a thick hypoechoic cortex (Figs. 11.1 and 11.2) (Siegel 2002a, b).
With involution, the foetal cortex is replaced by fibrous tissue. With age, the echogenicity of the gland increases, and the size decreases. During the end of the first year of life, the organ appears hyperechoic without clear margins between medulla and cortex.
During the following months and years, the fibrous tissue disappears and the organ gets the hypoechoic appearance of the older child. The triangular- or crescent-shaped structure in the suprarenal area of older children is caused by perirenal fat and not by the adrenal gland itself.
Size: The size of both adrenal glands does not vary significantly.
In neonates the length of the organ varies from 0.9 to 3.6 cm (mean 1.5 cm), and the thickness measures between 0.2 and 0.5 cm (mean 0.3 cm) (Oppenheimer et al. 1983; Scott and Thomas 1990). The postnatal involution causes a decrease in size of 40–50 % in the first 6 weeks of life (Scott and Thomas 1990).
In older children the adrenal glands measure 4–6 cm in length, 2–3 cm in width and 0.2–0.6 cm in thickness (Yeh 1987).
Doppler Sonography
The perfusion of the normal adrenal glands can routinely not be shown by colour or power Doppler. Under pathologic circumstances, such as enlargement of the adrenal gland and in all space-occupying lesions of the adrenal gland, the different Doppler techniques are indicated.
11.3 Pathologic Indications
11.3.1 Congenital Adrenal Hyperplasia
Congenital adrenal hyperplasia is suspicious of adrenogenital syndrome (AGS), a metabolic disorder of the adrenal gland. Several inborn errors in enzymatic production of cortisol and aldosterone cause adrenal hyperplasia by excessive ACTH stimulation. On sonography the adrenal glands may be enlarged (Fig. 11.4) (Bryan et al. 1988; Sivit and Hung 1991; Hernanz-Schulman et al. 2002). A mean adrenal length of 20 mm and a mean width of 4 mm or greater are suspicious of the diagnosis (Fig. 11.4) (Sivit and Hung 1991). The enlarged glands keep their triangular appearance and their normal corticomedullary differentiation (Fig. 11.4). Doppler sonography is usually not indicated in patients with AGS and provides no additional information.


Fig. 11.4
Hyperplasia of the adrenal gland in a neonate with adrenogenital syndrome. The enlarged adrenal gland (asterisks) has a thickness of 5 mm. The picture shows that all layers are hyperplastic. SC spinal cord
11.3.2 Space-Occupying Lesions of the Adrenal Gland
Space-occupying lesions of the adrenal gland in the neonatal period have to be differentiated in two major entities: adrenal tumours or adrenal haemorrhage (Fig. 11.5). The differentiation between adrenal haemorrhage and congenital neuroblastoma with 2D ultrasound alone may be difficult in the individual case.


Fig. 11.5
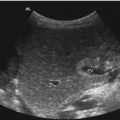
Space-occupying echogenic suprarenal lesions which displace the kidney caudally. (a) Neuroblastoma. Large echogenic suprarenal mass (markers) with compression of the upper pole of the kidney (K). The echogenic mass has inhomogeneous internal echoes and a central cystic area. L liver. (b) Adrenal haemorrhage in a newborn girl with an age of 8 days. The adrenal haemorrhage (markers) displaces the kidney (K) anteriorly. The haemorrhage is echogenic with heterogeneous internal echoes. L liver. (c) Adrenal haemorrhage. Large hyperechoic suprarenal mass with several cystic areas (markers). K kidney
2D Image
Both diseases compress the upper pole of the ipsilateral kidney and displace it laterally and downwards (Fig. 11.5). Sometimes the kidney is also displaced anteriorly (Fig. 11.5b). Usually congenital neuroblastomas and adrenal haemorrhages present as an echogenic suprarenal mass with heterogeneous echogenicity (Fig. 11.5). In rare cases congenital neuroblastomas may be predominantly cystic and adrenal haemorrhages may have a completely solid appearance (Fig. 11.5b) (Deeg et al. 2007). This makes the differentiation very difficult in the individual case.
Serial sonographic controls may be helpful for the differential diagnosis. Adrenal haemorrhages diminish in time, whereas neuroblastomas usually keep unchanged or get greater. The usual course of a congenital neuroblastoma is gradual shrinkage before it completely involutes within several months.
The differentiation between both entities is possible by colour Doppler or power Doppler (Deeg et al. 1998).
11.3.3 Adrenal Haemorrhage
Adrenal haemorrhages are typically found in large newborns after difficult often traumatic delivery. They may present with anaemia and hyperbilirubinaemia. Sonography detects a suprarenal mass of different size and echogenicity (Figs. 11.5b, c, 11.6a, c, 11.7a and 11.8a, b).




Fig. 11.6
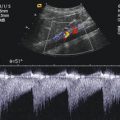
Adrenal haemorrhage in a 2-day-old boy. (a, c) Hypoechoic suprarenal mass with few internal echoes. The mass is surrounded by a thin echogenic rim of normal adrenal tissue. (a) longitudinal scan; (c) transverse scan. (b, d) Colour Doppler shows no internal vessels in contrast to good vascularisation of the kidney and liver. (b) longitudinal scan; (d) transverse scan

Fig. 11.7
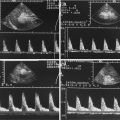
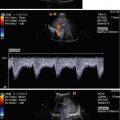
Adrenal haemorrhage in a newborn girl aged 7 days. (a) Longitudinal scan through the right kidney. The image shows a space-occupying lesion at the upper pole of the right kidney. The lesion displaces the kidney downwards and anteriorly. The echogenicity is similar to the kidney. Cystic areas cannot be seen. (b, c) Colour Doppler of the flow in the adrenal haemorrhage shows no internal vessels in contrast to the prominent vasculature of the adjacent kidney and liver. (b) longitudinal section; (c) transverse section. (d) Transverse section through the right kidney shows prominent internal vascularisation in contrast to the absent vessels of the adrenal hemorrhage. (e) Power Doppler of the perfusion of the haemorrhage. No perfusion can be shown in contrast to the kidney. (f) Pulsed Doppler recording of the flow in the adjacent renal artery shows normal flow profile and normal flow velocities

Fig. 11.8
Adrenal haemorrhage in a female neonate. (a) Longitudinal image on day 1: the haemorrhage has an echogenic appearance with small cystic areas in the border zone of the haemorrhage. (b) Transverse image through the upper pole of the right kidney on day 1: the haemorrhage covers the kidney cap like. (c, d) Colour (c) and power (d) Doppler at day 9 (d) and day 15 (c) shows missing perfusion of the haemorrhage in contrast to the perfusion of the kidney and liver. (e) Spectral Doppler of the flow in the renal artery shows decreased diastolic flow, due to compression of the artery by the adrenal hemorrhage
2D Image
The typical sonographic appearance of an acute adrenal haemorrhage is a round or triangular shaped suprarenal mass, replacing the entire adrenal gland (Figs. 11.6a, 11.7a, and 11.8a). Large adrenal haemorrhages displace the kidney downwards (Figs. 11.5b, c and 11.7a).
They may appear as echofree cystic masses (Fig. 11.6a, c) or may show fine internal echoes (Fig. 11.6c). With high-resolution linear transducers of 13 MHz or more, the adrenal cortex and the bleeding into the medulla can be shown (Fig. 11.6a, c). In rare cases adrenal haemorrhage may appear as a solid mass mimicking a tumour (Figs. 11.5b, c and 11.7a).
The echogenicity of the haemorrhage is dependent on the age of the haemorrhage. Acute haemorrhage is hyperechoic, in comparison with the normal gland and the surrounding tissue (Fig. 11.7a). Within the next days and weeks, the blood lyses and coalesces and the haemorrhage develops a hypoechoic centre (Fig. 11.6a, c). During the next weeks the cystic mass involutes and eventually disappears. The typical adrenal haemorrhage is initially often echogenic before it becomes more and more cystic and gradually decreases in size and disappears (Deeg et al. 2007).
Neuroblastomas on the other hand usually do not decrease in size within several weeks and can therefore be distinguished from adrenal haemorrhage. Although the differentiation between both entities is difficult in the individual case, an initially echogenic mass which gradually shrinks in size and becomes more and more hypoechoic is likely to represent resolving adrenal haemorrhage rather than a neuroblastoma (Deeg et al. 2007).
Congenital neuroblastoma usually has a good prognosis and may involute and disappear within the first 3 months of life.
Doppler Sonography
Colour Doppler and power Doppler do not show internal vessels (Figs. 11.6b, d, 11.7b, c, d, e and 11.8c, d). For the demonstration of a possible existing internal flow, high-resolution linear transducers and sensitive flow settings have to be chosen (Figs. 11.6b, d, 11.7b, c, d, e and 11.8c, d). The Nyquist limit should be so low that the slowest flow velocities can be measured. The lowest wall filter should be used. If appropriate settings are chosen, no internal flow can be shown in the case of adrenal haemorrhage (Figs. 11.6b, d, 11.7b, c, d, e and 11.8c, d). Power Doppler is probably the most sensitive Doppler method for the demonstration of missing flow (Figs. 11.7e and 11.8d).
11.3.4 Adrenal Cyst
Adrenal cysts can rarely be found in neonates and infants.
They are round- or oval-shaped echofree space-occupying lesions in the region of the adrenal gland (Fig. 11.9a, c). Colour Doppler shows no internal vessels (Fig. 11.9c). Adjacent vessels of the adrenal gland are displaced and compressed (Fig. 11.9c). Sometimes haemorrhage into the cyst occurs before the cyst ruptures and resolves (Fig. 11.9d).


Fig. 11.9
Congenital adrenal cyst in a female neonate. (a, b) The images show a cystic lesion without internal echoes at the upper pole of the kidney. (a) transverse; (b) longitudinal section through the right upper abdomen. (c) Colour Doppler shows no perfusion of the cyst. (d) Colour Doppler of the cyst with an age of 1 month. No internal perfusion in contrast to the kidney. The internal echoes in the cyst are possibly caused by secondary haemorrhage.
11.3.5 Neuroblastoma
Neuroblastomas are the most common malignant tumours of the abdomen and chest in infancy and childhood. The overall incidence of neuroblastomas is only 1.1/100,000 in children under 15 years of age (Kaatsch and Spix 2004).
Apart from the adrenal gland, neuroblastomas can originate all along the sympathetic chain from the brain to the pelvis. The majority of tumours however are abdominal in origin.
Forty percent arise from the adrenal medulla and 25 % from the intraabdominal and mediastinal sympathetic ganglia (Fig. 11.13) (Aaberg and Koyle 1991). Tumours arising from the posterior mediastinum may compress the bronchi and cause dyspnoea, cough, wheezing and respiratory emergency (Fig. 11.13a) (Loh and Matthay 2005). Tumours originating from the paravertebral sympathetic ganglia have a tendency to invade the intervertebral foramina, causing spinal cord compression and resultant neurological problems (Fig. 11.14) (De Bernardi et al. 2001; Holgersen et al. 1982; Kaatsch and Spix 2004).
11.3.5.1 Congenital Neuroblastoma
In the first year of life, the incidence of neuroblastoma is significantly higher with 6.4/100,000 (Kaatsch and Spix 2004). Clinically detected neuroblastoma is noted in only 1 per 10,000 to 1 per 100,000 live births (Aveci and Weinstein 1994).
Sixty percent of all neuroblastomas occur before the age of 1 year and 20 % before the age of 6 months (Loh and Matthay 2005; Altmann 1992). Neonatal neuroblastomas, however, are very rare. They represent only 9 % of all neuroblastomas (Berthold 2004). Congenital neuroblastomas account for 50 % of all malignancies which are diagnosed in the neonatal period (Aaberg and Koyle 1991; Chatten 1995). In the German neuroblastoma study, a total number of 3,130 infants with neuroblastomas were registered till September 2005 (Berthold 2004). In 283 patients, the tumour was diagnosed in the neonatal period.
2D Image
In most cases, a suprarenal or paravertebral space-occupying lesion displacing the kidney laterally and downwards can be shown (Figs. 11.10 and 11.11) (Amundson et al. 1987; Deeg et al. 1998; Lanning and Lanning 1992; Siegel 2002a, b; Swischuk 2004).



Fig. 11.10
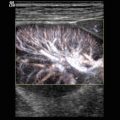
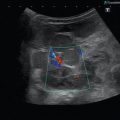
Congenital neuroblastoma in a 6-day-old girl. (a) Longitudinal scan through the right upper abdomen. Suprarenal mass (asterisks), which cannot clearly be delineated from the adjacent liver and kidney (K). The tumour has inhomogeneous internal echoes with focal areas of increased echogenicity and some small cysts. (b) Transverse section through the right upper abdomen. The image shows a tumour (markers) in the region of the renal hilus. The internal echoes are inhomogeneous and contain some cysts. (c) Colour Doppler of the flow in the suprarenal tumour. The image shows vascularisation of the outer portions of the tumour. The internal area including the cysts is not vascularised

Fig. 11.11
Congenital neuroblastoma in a girl with an age of 5 weeks. (a) Longitudinal scan through the right upper abdomen shows a good delineated suprarenal mass with inhomogeneous internal echoes. The tumour compresses the upper pole of the kidney (arrows). (b) Transverse scan through the tumour with a central cystic area and otherwise inhomogeneous echogenic outer cortex (asterisk). L liver. (c, d) Colour-coded Doppler sonogram shows vascularisation of the tumour with an irregular course of the pathologic vessels. The cysts represent areas of necrosis or haemorrhage and show no vascularisation. L liver
Normally, neonatal neuroblastomas appear as solid lesions (Figs. 11.10 and 11.11) (Forman et al. 1990).
In the neonatal period calcifications are unusual, whereas cystic areas may be seen which only rarely occur in older children (Siegel 2002a, b). In neonates the tumour can be markedly hypoechoic and may even appear cystic which makes the differentiation from adrenal haemorrhage difficult (Atkinson et al. 1986; Cassady and Winter 1997; Croitoru et al. 1992; Richards et al. 1995; Weber et al. 1993).
In the neonatal period, neuroblastomas originating from the adrenal glands have to be distinguished from adrenal haemorrhage. This may be very difficult by two-dimensional ultrasonography alone. In rare cases, congenital neuroblastomas may be predominantly cystic and adrenal haemorrhage may have a completely solid appearance (Atkinson et al. 1986; Cassady and Winter 1997; Croitoru et al. 1992; Hendry 1982; Lee et al. 1998; Richards et al. 1995). This makes the differentiation of both entities very difficult in the individual case. An important ultrasonographic characteristic of a cystic neuroblastoma may be a thick complex wall which cannot be found in adrenal haemorrhage (Atkinson et al. 1986; Cassady and Winter 1997; Croitoru et al. 1992). Serial sonographic controls may be helpful for the differential diagnosis. Initially, adrenal haemorrhages are often echogenic before they become more and more cystic and gradually decrease in size and disappear (Deeg et al. 1998; Gotoh et al. 1989; Lanning and Lanning 1992; Siegel 2002a, b; Swischuk 2004).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree