Fig. 7.1
Normal anatomy of the coeliac trunk and its branches. The transverse section through the upper abdomen shows the aorta, coeliac trunk and its main branches, the hepatic and the splenic artery as indicated. AO aorta, IVC inferior vena cava, HA hepatic artery, SA splenic artery, PV portal vein. The markers are set at the coeliac trunk
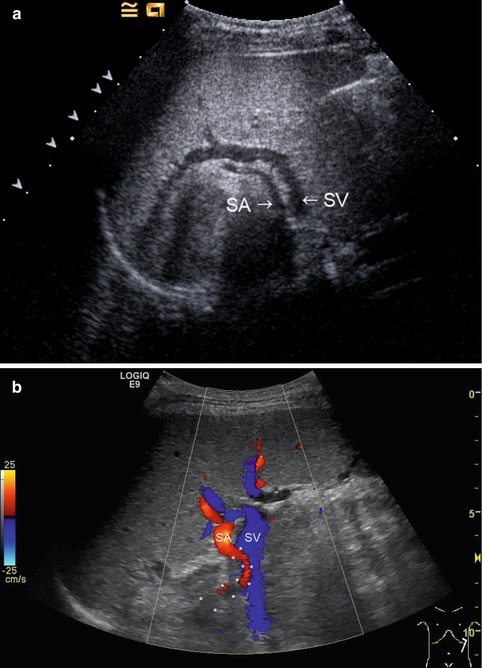
Fig. 7.2
(a) The splenic artery (SA) is located more cranially than the splenic vein (SV). Along the course of the splenic vein, the tail of the pancreas can be visualised. Oblique intercostal section through the left lateral upper abdomen. (b) Colour-coded Doppler sonography of the vascular supply of the spleen. Slightly serpentinous course of the splenic artery (red) in a 17-year-old adolescent with primary biliary sclerosis (the course of the splenic artery (SA) is marked with *). The splenic vein (SV) is displayed blue. Oblique intercostal section through the left lateral upper abdomen

Fig. 7.3
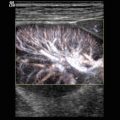
Colour (a) and power (b) Doppler of the spleen. The splenic artery divides within the spleen into segmental, subsegmental and subsubsegmental arteries, which extend to the splenic capsule. Longitudinal (a) and transverse (b) section through the spleen
The splenic vein drains the blood from the spleen in 2–6 branches at the hilus and forms, together with the superior mesenteric vein, the portal vein at the splenoportal confluence. The inferior mesenteric vein is the biggest vein which drains into the splenic vein (variance of 30 % into the superior mesenteric vein). Furthermore, the left gastric vein, the small gastric veins (Vv. gastricae breves) and the gastroepiploic veins drain into the splenic vein. The inflow of the inferior mesenteric vein into the proximal pancreatic part of the splenic vein can only rarely be visualised by ultrasonography.
7.2.2 Ultrasonographic Approach
The easiest approach for visualisation of the splenic vessels is a transverse section through the upper abdomen. The artery runs at the superior border of the pancreas, usually localised more cranially than the vein. The middle portion of the artery can also be shown in longitudinal sections through the upper abdomen with the transducer slightly angled to the left side (Fig. 7.4a–f). The artery is always neighboured by the splenic vein, which can be imaged behind the pancreatic corpus and tail in transverse and longitudinal sections (Fig. 7.5a–d). Using B-mode ultrasonography (US) alone, both vessels cannot be exactly distinguished. With the help of the different Doppler techniques (colour or spectral Doppler), however, artery and vein can easily be differentiated. From the left lateral intercostal approach, the splenic hilus and the intrasplenic vessels can be demonstrated (Fig. 7.6a, b).








Fig. 7.4
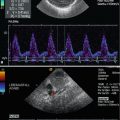
Course of the splenic artery from its origin from the coeliac trunk (CT) to the splenic hilus. SA splenic artery, PV portal vein, DAO descending aorta. Cross section. (a) 2D image of the proximal splenic artery in a 16-year-old boy. (b) Colour Doppler: the coeliac trunk is displayed red, the splenic artery blue. (c) Spectral Doppler of the flow in the proximal splenic artery shows a systolic-diastolic forward flow which is displayed below the baseline, as the flow is directed away from the transducer. (d) Colour Doppler of the flow in the splenic artery (SA) in a transverse section through the middle upper abdomen. rRA right renal artery, DAO descending aorta. (e) Spectral Doppler of the flow in the distal splenic artery at the splenic hilus. The flow curve is displayed above the baseline, as the flow is directed towards the transducer and the spleen. (f) The middle portion of the splenic artery can also be shown in longitudinal sections with the transducer slightly angled to the left side. The flow in the splenic artery in this section is directed away from the transducer and therefore displayed blue and depicted below the baseline in the PW-Doppler. The PW-Doppler shows a systolic-diastolic forward flow due to low peripheral resistance



Fig. 7.5
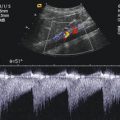
(a) The splenic vein can be imaged behind the body and tail of the pancreas in B-mode. Transverse section through the upper abdomen. (b) Colour Doppler of the flow in the splenic vein in a transverse section through the upper abdomen. The flow in the splenic vein (SV) along the pancreatic tail is directed towards the transducer and therefore displayed red. Close to the pancreatic head, the flow in the splenoportal confluence (SPC) is directed away from the transducer and therefore displayed blue. The aorta (DAO), the inferior vena cava (IVC), right renal artery (RA) and the superior mesenteric artery (SMA) are shown. (c) Spectral Doppler of the flow in the splenic vein shows an antegrade flow with a time average maximal velocity (TAMAX) of 30.7 cm/s and a mean time average velocity (TAMEAN) of 16.6 cm/s. Transverse section through the upper abdomen. (d) Longitudinal section through the middle upper abdomen shows the pancreatic body (P) and the splenic vein (SV). The stomach is marked with “S”

Fig. 7.6
(a ) B-mode section through the splenic hilus. The length of the spleen is measured. The tail of the pancreas can be visualised caudally to the splenic vein (P). The splenic artery (SA) is smaller in size and located more cranially than the splenic vein (SV). Left lateral intercostal approach. (b) Colour Doppler of the flow in the splenic hilus. The flow in the splenic vein (SV) is directed away from the transducer and coded in blue; the flow direction in the splenic artery (SA) is towards the transducer and therefore coded in red colour. Left lateral intercostal approach
The investigation of the spleen can be performed non-fasting and without special preparation in the supine position or lying on the right side.
Transducers: The appropriate transducer frequency has to be chosen depending on the age/size of the patient and the indication. In neonates, infants and for the detection of smaller focal lesions, high-resolution linear transducers up to 15 MHz are optimal (Fig. 7.7a, b). Older children, especially obese adolescents, are best investigated with a curved array transducer <6 MHz, followed by an additional approach with a high-frequency transducer in order to detect smaller lesions, discontinuation of the splenic surface in cases of trauma and superficial minor vascular pathologies, which may not be detected with low-frequency curved array scanning (Fig. 7.9).


Fig. 7.7
Newborn with hepatosplenomegaly due to myelodysplastic syndrome. In newborns and infants and for the detection of smaller focal lesions, high-resolution linear transducers up to 17 MHz are optimal for visualisation of the parenchyma. (a) “Kissing phenomenon” of the liver and spleen. The stomach is in between. B-mode image. Transverse section through the spleen. (b) Colour Doppler image of the splenic perfusion. Transverse section through the spleen
7.2.2.1 Colour and Pulsed Doppler Sonography (CDS and PW-DS)
Using colour-coded Doppler sonography (CDS) in a transverse mid-abdominal section, the flow in the splenic artery behind the pancreas can be displayed blue as the flow is directed away from the transducer (Fig. 7.4b, d). This translates into a negative flow in the splenic artery in pulsed waved (synonym: spectral) Doppler sonography (PW-DS) behind the pancreatic body (Fig. 7.4c, f) and a positive flow after the artery has passed the pancreatic tail (Fig. 7.4e).
In the splenic vein, however, the flow behind the body of the pancreas is directed towards the transducer and is therefore displayed red (Fig. 7.5b). Pulsed Doppler shows a forward flow displayed above the baseline (Fig. 7.5c). Similar to the flow in the portal vein, a nearly continuous positive flow with small undulations can be seen (Figs. 7.5c and 7.8).


Fig. 7.8
Pulsed-wave Doppler sonography in the splenic vein with a laminar flow profile. Transverse section through the spleen
The main indications for Doppler sonography (DS) of the spleen are listed in Table 7.1.
Table 7.1
Main indications for Doppler sonography (DS) of the spleen
Differential diagnosis of splenomegaly (acute and chronic infections, haematological and immunological diseases, portal hypertension, storage diseases) |
Differential diagnosis of reduced splenic size (hyposplenia/asplenia) |
Diffuse alterations of the spleen (diffuse benign or malign infiltration, systemic inflammatory or infectious diseases) |
Vascular alterations (thrombosis, infarction, aneurysm) |
Trauma |
Focal lesions of the spleen |
7.3 Splenic Trauma
The spleen is often injured in accidents, although it is shielded by the ribs of the left hemithorax, which usually protects the spleen from laceration. Enlarged spleens which tower below the costal arch are extremely vulnerable. In blunt abdominal trauma, injury of the spleen must always be ruled out as the spleen is the most common injured abdominal organ accounting for up to 45 % of all visceral injuries (Tataria et al. 2007). An enlarged spleen is far more at risk of being injured after blunt abdominal trauma. Sonographic signs of possible spleen injury are free abdominal fluid with internal echoes, splenomegaly, reduced respiratory movement, inhomogeneous texture of the spleen, subcapsular haematoma, anechoic lacerations within the parenchyma or disruption of the splenic capsule (Hofmann 2005) (Figs. 7.9a–d and 7.10a, b). In many emergency rooms, a FAST (focal abdominal sonography for trauma) protocol with scanning of the four abdominal quadrants for free fluid, as a sign for haemoperitoneum, is done and later followed by a complete and detailed US investigation after deciding on surgical or conservative therapy for a trauma patient. However, in a study by Richards in 2002, 12 % of children with abdominal injury had no free fluid. Contusions, lacerations, ruptures, fractures, subcapsular and perisplenic haematoma (Figs. 7.9b and 7.10a) of the spleen may be distinguished. Haemoperitoneum and active bleeding are the most likely indications for surgical intervention. Due to the thicker splenic capsule in children, operations in splenic trauma are less frequently compared to adults. In paediatric patients, haemodynamic stability is the most important determinant for conservative treatment. Following trauma, splenic healing can rapidly be observed. This was experienced in the last decades of predominately successful conservative management of splenic injuries. Because of devastating post-splenectomy infections with encapsulated bacteria such as Streptococcus pneumoniae, Neisseria meningitidis and Haemophilus influenzae, splenectomy should be avoided whenever possible.



Fig. 7.9
(a) Splenic rupture in a child with a traffic accident. The B-mode image shows a hypoechoic rectangular area marked with *. Transverse section through the spleen. (b) Contusion of the apical splenic area in an 8-year-old child after bicycle accident with blunt abdominal trauma. Around the apex of the spleen, a hyperechoic delineation of a perisplenal haematoma can be seen. (c) Same patient. CDS shows only the perfusion of major vessel branches in the central part of the spleen, but no perfusion within the region of contusion. (d) Same patient. An echoic rim of blood is located between the spleen and the stomach wall in a B-mode scan, oblique section

Fig. 7.10
Splenic trauma with a fracture through the lower pole and gross perisplenic haematoma. (a) B-mode. An echogenic band of blood is seen between spleen and kidney and echofree/echorich haematoma all around the splenic borders. Longitudinal section through the spleen. (b) CDS shows perfusion only in the dorsal parts of the spleen. CDS motion artefacts on the ventral side are marked with *. Longitudinal section through the spleen
Long-term sequelae of splenic injury may be scarring of parts of the spleen, development of posttraumatic pseudocysts, polysplenia and calcification (Fig. 7.11). In 15–30 % of patients, a two-phase delayed splenic rupture may be expected within 2 weeks. Delayed complications, such as splenic abscesses and pseudoaneurysms of the splenic artery and its branches, have been observed (Lynn et al. 2009). To detect complications, a short follow-up by B-mode sonography and CDS should be scheduled (Goletti et al. 1996). In cases of suspected splenic injury, CDS with low flow settings and a low wall filter should be used (Fig. 7.12a). If a high-frequency curved array probe is used, power Doppler is especially helpful for the diagnosis of focal hypoperfusion of the organ (Fig. 7.12b). Areas without perfusion can be distinguished from the surrounding normal vascularised parenchyma. Lacerations are displayed as focal hypoechoic lesions. Power Doppler reveals missing perfusion, often in a triangular shape. Acute posttraumatic injury and lacerations of the spleen may not be visualised with B-mode due to diffuse swelling of the organ. In the detection of traumatic splenic injury, CEUS (contrast-enhanced ultrasonography) is much more sensitive than mere B-mode and Doppler US alone (see Sect. 7.8, Fig. 7.13; Manetta et al. 2009; Weskott 2013).




Fig. 7.11
Traumatic polysplenia. Four splenic residuals are left in this child after a traffic accident. Longitudinal section through the spleen

Fig. 7.12
Trauma of the spleen. (a) Due to diffuse haematoma and splenic contusion, the colour-coded Doppler sonogram shows no vessels in the lower pole of the spleen, intercostal oblique section. (b) Power Doppler reveals no perfusion in the traumatised triangular subcapsular splenic area. Transverse section through the spleen

Fig. 7.13
Contrast-enhanced ultrasonography (CEUS) in an adolescent with two parallel lacerations of the spleen after a traffic accident. Subphrenic haematoma around the spleen. Intercostal oblique section
7.4 Focal Lesions of the Spleen
7.4.1 Introduction
Focal lesions of the spleen are rare (0.2 %, Bachmann and Görg 2005; Chen 2005; Görg 2011). Bachmann et al. described 98 adult patients with focal lesions and classified them as avascular, hypovascular, isovascular, hypervascular and arteriovenous “high flow”, using the surrounding splenic tissue as in vivo reference. Most of the focal splenic lesions (68.4 %) were avascular, 15.3 % appeared hypovascular, 8.2 % isovascular, 5.1 % hypervascular and in 3.1 % an arteriovenous “high-flow” pattern was found. Alongside the diagnosis of intrasplenic pseudoaneurysm, the practical utility of CDS in differentially diagnosing focal spleen lesions is low (Bachmann and Görg 2005). However, US has a role in the detection and follow-up of focal lesions. Cystic and solid lesions are distinguishable in B-mode scanning.
7.4.2 Benign Splenic Lesions
7.4.2.1 Cystic Lesions
Cystic lesions are most commonly dysontogenetic (simple cysts) followed by non-epithelialised pseudocysts (synonym: secondary cyst) and parasitic cysts (hydatid cyst, usually caused by Echinococcus granulosus).
Congenital, dysontogenetic cysts (synonyms: simple cyst, real cyst, epidermoidal cyst, primary cyst) are usually anechoic, round and solitary. They usually show a hyperechogenic epithelial lining at their borders to the splenic parenchyma (Fig. 7.14a, b), a dorsal acoustic enhancement and only occasionally thin septa.


Fig. 7.14
Two simple cysts in a 5-week-old infant as an accidental finding (synonyms: dysontogenetic, simple, real, epidermoidal, primary cyst). (a) B-mode with a high-frequency 14 MHz probe. Transverse section. (b) Colour-coded Doppler sonogram (CDS) of the same patient. The two dysontogenetic cysts show no internal Doppler signal. Vessels can only be displayed in the normal neighbouring parenchyma. Transverse section
Secondary cysts are diagnosed following trauma, infection or infarction. They are mostly not completely round; multiple and intraluminal septa are present more frequently. Doppler sonography shows no signals within cysts (Fig. 7.15a, b). The difference between a primary and a secondary cyst depends mainly on the presence of an epithelial wall surrounding the cyst. The differentiation is not always reliable with US.


Fig. 7.15
An 8-year-old girl with upper abdominal pain and incidental finding of a splenic cyst. (a) B-mode US shows an irregularly delineated cyst measuring 2.5 cm in diameter. Fine echoes can be seen within the cyst and a small parenchymal tongue at the border. Longitudinal section. (b) Colour-coded Doppler sonogram (CDS) shows no signals within the cyst, but vessels can be displayed in the neighbouring parenchyma. Longitudinal section
The intracystic fluid of secondary cysts may have an increased echogenicity due to cholesterol crystals, inflammatory debris or haemorrhage. Parietal calcifications are more common in pseudocysts. Surgical enucleation of large cysts may be necessary, but procedures should always be organ preserving (Czauderna et al. 2006). Complications of nonparasitic splenic cysts include intracystic haemorrhage, rupture, peritonitis and hypersplenism (Czauderna et al. 2006).
Echinococcal parasitic infections have to be considered in the differential diagnosis of cystic lesions of the spleen, although splenic involvement is rarer than hepatic or pulmonary manifestations (Fig. 7.16). Splenic echinococcal involvement is reported only in 0.9–8 % of patients, usually in infants with multi-organ disease.


Fig. 7.16
Echinococcal cyst of the spleen (E. granulosus). The image shows multiple internal echoes, pseudomembranes and debris within the cyst. Longitudinal section
Isolated splenic parasitic cysts are even rarer. In early stages of echinococcal infection, a hyperperfused rim may be seen with DS. Hydatid sand, daughter cysts and infolded membranes may be visible within the cyst, but they can also appear purely cystic or solid. In later stages, calcifications may occur (Dilli et al. 2011). Another cause of parasitic cysts is porcine tapeworm (Taenia solium) infections (Benter et al. 2011).
Lymphangiomas consist of multiple vascular canals filled with lymphatic fluid. Therefore, they have a hypo- or anechoic, pluriseptate appearance with possible debris or calcification inside (Fig. 7.17a, b). Usually, no vessels can be detected inside lymphangiomas. In rare cases however, vessels can be seen within the septae of a lymphangioma. Lymphangiomas grow slowly. They may appear single or multiple (Paterson et al. 1999), mostly with a multicystic, “honeycomb” appearance, rarely like a solid tumour or a solitary cyst. Unlike haemangiomas, the capsule and trabeculae of the spleen, where lymphatic tissue is concentrated, are often involved. The clinical manifestation may range from a small incidental lesion to a large multicystic abdominal mass requiring surgical intervention (Abbott et al. 2004). In lymphangiomatosis, multiple organs are involved, most often the liver, mediastinum, axilla, neck and retroperitoneum.


Fig. 7.17
Lymphangioma of the spleen. Lymphangiomas consist of multiple vascular canals filled with lymphatic fluid. (a) B-mode: multiple anechoic round lesions with thin septation. Usually no vessels can be detected inside the lymphangiomas. Transverse section. (b) Multiple cystic lesions which are separated by thin septations. Longitudinal section through the spleen. (c) Abdominal MRI of the same patient with lymphangioma of the spleen
7.4.2.2 Solid Lesions
Solid lesions show echoes within the lesions and may show perfusion in CDS and pulsed Doppler sonography. In CDS two-thirds of focal splenic lesions appear to be avascular (Bachmann and Görg 2005). The importance of imaging solid splenic lesions is to differentiate them from malignant lesions. For all imaging modalities (US, CT, MRI, CEUS), a definite differential diagnosis of solid splenic focal lesions is difficult.
Although rare, the most frequent benign solid splenic lesions are haemangiomas.
Haemangiomas usually appear hyperechogenic and in CDS often without signals (Taibbi et al. 2012). Their appearance in the spleen is non-specific on B-mode and CDS. Depending on the vessel size inside the haemangioma, capillary and cavernous types can be distinguished. The cavernous type has a combination of solid and cystic components and appears more sponge-like. Haemangiomas may occur solitary or multiple and are found more frequently in association with Klippel-Trénaunay-Weber, Turner or Beckwith-Wiedemann syndromes. Splenic haemangiomas are usually asymptomatic, but complications include bleeding, rupture and Kasabach-Merritt syndrome, a life-threatening consumptive coagulopathy due to disseminated intravascular coagulation starting in the abnormal haemangioma vessels with thrombocytopenia and anaemia (Peddhu et al. 2004). Most lesions are small and found incidentally in asymptomatic patients (Abbott et al. 2004); calcifications may occur.
CDS may reveal flow within the haemangioma; however, splenic haemangiomas often do not show the typical rim enhancement with centripetal inflow into the centre of the lesion as seen in hepatic haemangiomas especially in contrast-enhanced ultrasonography (CEUS) (Taibbi et al. 2012). Capillary haemangiomas and hamartomas seem to be the most likely diagnosis in hyperechogenic lesions, but in all cases a careful ultrasonographic follow-up is warranted (Görg et al. 2006). Even pathological differentiation of hamartomas from haemangiomas may be difficult in the individual case (Abbott et al. 2004).
Hamartomas (splenoma, splenadenoma or “FNH of the spleen”) are non-neoplastic lesions, which are composed of different splenic components apart from the white pulp. The hamartoma/splenoma was first described by the pathologist von Rokitansky in 1861. It consists of irregular red pulp with sinusoidal-like vessel structures and fibrous tissue (Günter 2005; Hartmann et al. 2008; Abramowsky et al. 2004), therefore also called “focal nodular hyperplasia (FNH) of the spleen”. They are typically solitary and asymptomatic; calcifications can be found. Their size varies up to 20 cm (Hartmann et al. 2008) and splenomegaly is usually present. CDS may depict hypervascularity with multiple intralesional colour flow signals distributed in a radial fashion (Görg and Schwerk 1994 and Peddhu et al. 2004). Hamartomas are usually well-described nodular lesions, which tend to compress the adjacent parenchyma. Calcification secondary to ischaemia or haemorrhage is rare.
Mostly the discovery of a hamartoma/splenoma is incidental; in one case out of 170 described, a spontaneous rupture occurred (Günter 2005). Hamartomas are thought to be congenital in origin, and splenic hamartomas have been associated with further hamartomas in other organs such as in patients with tuberous sclerosis or Wiskott-Aldrich-like syndromes (Abbott et al. 2004). However, the aetiopathology remains unclear.
Haemangioendotheliomas are benign vascular tumours with sometimes significant morbidity (organ enlargement, Kasabach-Merritt syndrome and congestive heart failure). The US appearance in the spleen is non-specific; they are mostly described as hypoechoic. Anechoic areas may represent intralesional necrosis compared to the normal splenic parenchyma. Haemangioendotheliomas are hypovascularised in CDS. Calcifications are not a specific feature.
More rare benign manifestations of focal splenic lesions are lipomas and angiomyolipomas (Fig. 7.18).


Fig. 7.18
An 8-year-old girl with tuberous sclerosis. Multiple angiomyolipomas are seen as hyperechoic roundish lesions within the kidneys and a few solitary echogenic lesions within the spleen. Longitudinal section
Littoral cell angioma of the spleen is a rare vascular tumour seen at any age with no gender predisposition. Originally, littoral cell angiomas were thought to be benign, but also malignant features have been described (Abbott et al. 2004). Splenomegaly is almost always present. Littoral cell angioma originates from the red pulp sinuses. In US, lesions are usually multiple. They may have the same echogenicity as the spleen or may be hyper- or hypoechoic. They may be innumerable and confluent, then appearing diffusely with heterogeneous echotexture of the spleen.
Langerhans cell histiocytosis with proliferation of bone marrow-derived histiocytes causes diffuse or focal hypoechoic nodules in the spleen, which vanish after therapy (Paterson et al. 1999) (Fig. 7.19a–c).