Fig. 1
Composition of the skin. (sq) squamous cells, (b) basal cell, (m) melanocytes, (f) fibroblast, (dc) dendritic cell, (lc) Langerhans cell, (mc) mast cell, (Mc) Merkel cell, (h) hair, (n) nerve, (P) Pacinian corpuscle, (v) vessels, (s) sebaceous gland, (mu) muscle, erector pili, (ec) eccrine gland, (Me) Meissner’s corpuscle. (Credited to MSKCC)
The thickness of the skin (epidermis and dermis) varies between 0.5 and 4 mm in locations such as the eyelids and palms/soles, respectively.
The skin covers almost all external surfaces of the body, for an average surface area of 1.79 m2 (men 1.91 m2, women 1.71 m2) (Sacco et al. 2010). Body surface area can be approximated by height and mass using the Dubois and Dubois method:


The palm of a patient’s hand is estimated to be approximately 0.5 % of the total body surface area (Rhodes et al. 2013).
The lymphatic channels of the skin are within the dermis and drain to lymphatic channels of the hypodermis and eventually to the regional lymph node basins. Note that some skin regions will drain to multiple lymph node basins.
An interactive tool is available to aid in the prediction of the draining lymph node basin for primary skin cancers of the head and neck (http://sites.bioeng.auckland.ac.nz/hrey004/head/) and trunk and extremities (http://sites.bioeng.auckland.ac.nz/hrey004/) (Figs.2, 3, 4, 5, and 6).






Fig. 2
Likelihood of lymphatic drainage to axillary lymph nodes from different sites of primary skin cancer (Reynolds et al. 2007). Drainage to left axillary lymphatics (a, b); and right axillary lymphatics (c, d). Regions of skin with no lymphatic drainage to axilla are colored black. (a, c) are anterior projections and (b, d) are posterior projections

Fig. 3
Likelihood of lymphatic drainage to the groin lymphatics from different sites of primary skin cancer (Reynolds et al. 2007). Drainage to left groin lymphatics (a, b); and right groin lymphatics (c, d). (a, c) are anterior projections and (b, d) are posterior projections

Fig. 4
Likelihood of lymphatic drainage to the left (a) and right (b) preauricular lymphatics from different sites of primary skin cancer (Reynolds et al. 2009)

Fig. 5
Likelihood of lymphatic drainage to the left (a) and right (b) upper cervical (level II) lymphatics from different sites of primary skin cancer (Reynolds et al. 2009)

Fig. 6
Likelihood of lymphatic drainage to anterior (preauricular, submental, cervical I–IV, a) or posterior (postauricular, suboccipital, cervical V, supraclavicular, b) neck lymphatics from different sites of primary skin cancer (Reynolds et al. 2009)
Different types of skin cancer have different proclivities for spread.
Local spread can occur superficially along planes of the skin and hypodermis (SCC, especially sclerosing or desmoplastic subtypes; BCC, especially infiltrative or morpheaform subtypes; angiosarcoma), along cutaneous nerves (SCC, BCC, and melanoma, especially desmoplastic and/or neurotropic subtypes), and through direct invasion of subcutaneous and deeper tissues (SCC, BCC, especially deeply invasive subtypes).
Regional spread can develop via “in-transit” dermal lymphatics to lymph node basins (Merkel cell carcinoma, melanoma, angiosarcoma, SCC, especially deeply invasive subtypes).
Distant spread can occur hematogenously (Merkel cell carcinoma, melanoma, SCC, especially those that have spread regionally).
2 Diagnostic Workup for Relevant Target Volume Delineation
2.1 Clinical Evaluation
Symptom assessment is critical to help determine if a skin cancer harbors high-risk features.
Local neurologic symptoms such as pain, paresthesias, anesthesia, dysesthesia, pruritus, and formications can herald neurotropic cancer and should be routinely queried and investigated further if present.
Physical examination for abnormal local neurologic signs in the case of potentially neurotropic skin cancers is essential. Strength, sensory, or reflex impairments should prompt further evaluation when abnormalities are present.
Examination of regional lymph node basins at risk for disease is also vital. Primary skin cancers at or approaching midline require examination of the lymph node basins bilaterally.
Direct visualization of the skin under bright light (projected at different angles) and magnification is the best means of determining the extent of superficial disease. Carefully observing the color, texture, creases, follicles, and hair growth pattern of the skin harboring cancer and comparing this to normal skin can be helpful.
Palpation of the skin can also help delineate the extent of superficial disease. For skin cancers on surfaces that can be palpated between gloved fingers from opposing surfaces (lip, ear, cheek), this practice is encouraged. Assess fixation to underlying structures by moving the lesion along the plane of the skin surface. Fixation of the skin cancer to the structures beneath subcutaneous tissues can suggest local invasion and requires further evaluation when present.
2.2 Imaging
Calibrated clinical digital photography is encouraged to characterize and record the appearance of skin cancer. Appropriate lighting, positioning, focus, exposure, and zoom are encouraged (Bhatia 2006).
Wood’s lamp (ultraviolet light at ~365 nm) can help delineate the extent of pigmented skin cancers (Paraskevas et al. 2005).
Investigational modalities for determining the superficial extent of skin cancer include, but are not limited to, reflectance confocal microscopy, high-frequency ultrasonography, optical coherence microscopy, and in vivo fluorescence microscopy.
Gadolinium-enhanced MRI can help assess the extent of perineural invasion (PNI) in cases of neurotropic skin cancer. Typically, patients with radiographic evidence of perineural spread will also have symptoms or signs of PNI (clinical PNI or cPNI) and pathologic evidence of PNI (pPNI). The absence of radiographic evidence of perineural spread does not exclude the possibility of neurotropic skin cancer.
CT provides a superior assessment of cortical bone and is the preferred modality for determining the extent of local bone invasion. MRI can identify marrow involvement.
MRI and CT with or without PET can all be valuable in the assessment of regional lymphadenopathy. Nodes are considered involved with cancer when they exhibit abnormal morphology (metabolically active, centrally necrotic, irregular capsule, rounded shape without fatty hilum) or size (>10 mm in short axis) (Schwartz et al. 2009).
2.3 Tissue Sampling
Punch, rather than shave, biopsy to the superficial reticular dermis through the tumor is the preferred method of diagnosis of skin cancer. Shave biopsy may be preferable in cosmetically sensitive areas or in certain patient populations; however, the depth of invasion and high-risk histopathologic features are often difficult to ascertain on shave biopsy.
Some “infiltrative” types of skin cancers are superficially occult. Mapping biopsies at the periphery of a poorly defined skin cancer can help determine the extent of disease not apparent on visual inspection.
Sentinel lymph node biopsy should be considered for several forms of skin cancer when there is no clinical evidence of regional lymph node metastasis: cutaneous melanoma >0.75 mm thick, all Merkel cell carcinoma (regardless of size or thickness), cutaneous SCC >2 mm thick.
3 Simulation and Daily Localization
3.1 General Guidelines
Patient positioning for the treatment of skin cancer is highly dependent on the location of the target volume and radiotherapy technique.
CT (with or without PET, with or without MRI) is performed for simulation. Intravenous contrast media is recommended for all sites, when not medically contraindicated. Oral contrast is recommended for patients with a target volume near the gastrointestinal tract. Slice thickness of 2 mm is preferred for target volumes incorporating cranial nerves or the skull base. Otherwise, a slice thickness of 3 mm is preferred. Head and neck scans should include the entire head and neck through the carina. Upper trunk and extremity scans should include the mastoid processes through the entire volume of the lungs. Lower trunk and extremity scans should include the iliac crests through the popliteal fossa. Isocenter is placed in the center of the target volume.
Image registration and fusion of MR and PET with CT should be performed to help guide target delineation or relevant targets (nerves, ganglia, hypermetabolic tumors).
3.2 Skin Surface Targets
Prior to simulation, patients should be reexamined and the superficial extent of the skin cancer marked on the skin surface with a single use sterile marker. Fiducial markers (radiopaque wires with adhesive) should be placed on these marks to aid in target volume delineation in CT datasets. Of note, patients with clinical or pathologic evidence of extranodal extension of carcinoma are at risk for dermal disease in proximity to any surgical scars. A 2 cm perimeter is marked around surgical scars, and this along with the surgical scars themselves (incision and drain sites) should be marked with fiducial markers. Prior radiotherapy sites should also be delineated on the skin surface using fiducial markers.
Patients with skin, in-transit lymphatics, or regional node basins at risk for dermal disease should have appropriate tissue equivalent bolus fabricated prior to fabrication of the immobilization equipment. Bolus thickness will depend on radiotherapy technique (generally, we use 0.5 cm for conformal plans incorporating beams oblique to the skin surface and 1.0–1.5 cm for single-field and parallel-opposed beam arrangements when beams are normally incident to the skin surface). Tissue equivalent bolus should be positioned between the skin surface at risk and the immobilization equipment. When possible, the tissue equivalent bolus should be fixed to the immobilization equipment to aid in setup reproducibility.
When the skin surface is at risk, in vivo dosimetry is encouraged to confirm adequate dose at the skin surface. This is particularly important with heavily modulated planning techniques (e.g., IMRT) (Price et al. 2006).
3.3 Head and Neck Targets
Patients with skin cancers of the head and neck are usually immobilized supine with a thermoplastic mask. In some cases, the prone position may be preferable for posterior targets. For the treatment of the neck lymphatics only, the neck is extended. For the treatment of cranial nerves V1 and V2 or the skull base, the neck is kept in a neutral position, with the hard palate normal to the table top. Gaze fixation and intraoral stent may also be valuable in this situation.
3.4 Upper Trunk or Extremity Targets
Patients with skin cancers of the upper trunk or extremities are immobilized supine in an alpha cradle. In some cases, the prone position may be preferable for posterior targets. For patients with a target volume in the axilla, the ipsilateral arm is placed akimbo. This position is preferred to arm maximally abducted as patients are often elderly and have very recently undergone axillary surgery, which limits the ability to abduct the arm. Absent these conditions, maximal arm abduction is an acceptable alternative position. CT bore and linear accelerator gantry permitting, the arm should be abducted enough so that no skin folds are present in the axilla. This helps reduce dermatitis in the axilla. Rotation of the arm and immobilization at the hand may be necessary for patients with target volumes along the length of the extremity or in the epitrochlear fossa.
3.5 Lower Trunk or Extremity Targets
Patients with skin cancers of the lower trunk or extremities are immobilized supine in an alpha cradle. In some cases, the prone position may be preferable for posterior targets. For patients with a target volume in the groin, the ipsilateral hip is abducted (“frog-leg”). CT bore and linear accelerator gantry permitting, the leg should be abducted enough so that no skin folds are present in the groin. This helps prevent dermatitis in the groin. Rotation of the leg and immobilization of the foot may be necessary for patients with target volumes along the length of the leg or in the popliteal fossa.
3.6 Daily Localization
Localization is dependent on the anatomic region being treated. Generally, for skin cancers of the head and neck, daily orthogonal kV images are used for localization. This is particularly important when targeting cranial nerves and the skull base. For skin cancers of the extremity and trunk, weekly MV images are used for localization.
4 Target Volume Delineation and Treatment Planning (Figs. 7, 8, 9, 10, and 11)

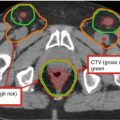
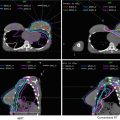
Fig. 7
Target delineation of CTVs in a 75-year-old man with rpT0N2bM0R0 cutaneous squamous cell carcinoma of the vertex of the scalp in axial CT displayed from cranial to caudal direction. He underwent wide excision of the primary tumor, adjuvant radiotherapy to the scalp vertex, and recurrence in left temporal in-transit and preauricular lymphatics. He underwent excision of the left temporal in-transit metastasis and left superficial parotidectomy. Pathology revealed squamous cell carcinoma within the left temporal dermis and parotid gland, not within a lymph node. Adjuvant radiotherapy was recommended to the left temporal in-transit lymphatics (orange, intact; dark blue, free flap), left preauricular (light blue), postauricular (yellow) and cervical (IB-VB, pink) lymphatics, and the facial nerve (green) to a dose of 60 Gy in 30 fractions, with a cone-down boost to the sites of resected carcinoma (turquoise and light blue) to a total dose of 70 Gy in 35 fractions over 7 weeks. Please note that these are representative images and not all images are included

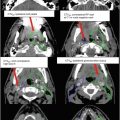
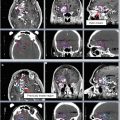
Fig. 8




Target delineation of CTVs in a 54-year-old woman with pT2N1aM0R0 Merkel cell carcinoma of the left posterior thigh in axial CT displayed from cranial to caudal direction. She has a history of renal allograft (note left pelvic kidney) and underwent wide excision of the primary tumor and sentinel lymph node biopsy of a left inguinofemoral lymph node. Pathology revealed a 4.5 cm Merkel cell carcinoma, with microscopic involvement of the sentinel lymph node. Adjuvant radiotherapy was recommended to the left posterior thigh primary tumor site (yellow), in-transit lymphatics (pink), and left inguinofemoral lymphatics (blue) to a dose of 50 Gy in 25 fractions, with a cone-down boost to the site of resected carcinoma to a total dose of 60 Gy in 30 fractions over 6 weeks. Please note that these are representative images and not all images are included
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree