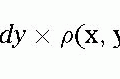Alternative Uses for Contrast Imaging
Robert W. W. Biederman
Standard clinical uses for contrast imaging by cardiovascular magnetic resonance (CMR) have been described in the earlier chapters. Generally, these pertain to the use of the vascular imaging, including aortic runoffs, and mesenteric and renal imaging. More recently, the use of contrast administration for the understanding of myocardial properties has shown major advantages. Historically, T1– and T2-weighted sequences, or variations on them, provided all of the contrast weighting possible. In Chapter 12 concerning the late hyperenhancement sequence, delayed uptake within the myocardium was demonstrated to accurately detect myocardial infarctions (MI’s). In this chapter, we will detail some of the more creative uses of contrast imaging for the identification of vascular, nonvascular, and myocardial properties.
MYOCARDITIS
Recently, the evaluation of myocarditis by CMR has become an important tool in nonischemic cardiomyopathies. In patients presenting with signs and symptoms of heart failure, but without demonstrable evidence of coronary artery disease (CAD), the search for a nonischemic mechanism is possible using CMR. The pattern of late hyperenhancement in these patients is distinctly different from the pattern characteristic of MI (see Fig. 15-1). Importantly, in cases of myocarditis it has been stated that late hyperenhancement is sensitive, but not specific. We note that the phenotype of the delay hyperenhancement distribution with relation to the myocardium gives clues concerning the underlying etiology. Whereas a MI always originates in the subendocardium, extending toward the midwall, and finally, if large enough, become completely transmural, myocarditis patterns are generally much more heterogeneous (see Fig. 15-2). Specifically, myocarditis patterns are generally less focal, much more patchy with skip lesions and, by definition, do not involve a coronary artery distribution (see Fig. 15-3). In some cases, the presentation of myocarditis can provide information about the long-term prognosis. This work is still maturing, but in general, larger, more focal patterns correspond with regional wall motion abnormalities, which will only marginally improve at 6 weeks, with residual contrast still present. Smaller lesions that are patchy or more diffuse, typically in concert with the clinical presentation, resolve in 6 weeks with a parallel improvement in left ventricular wall motion.
Most recently, several authors, including us, have made the interesting observation that the delayed hyperenhancement (DHE) signal in viral cardiomyopathy may demonstrate a linear stripe through the midwall of the myocardium with moderate signal intensity, which is generally a less intense signal than a DHE defined infarct (see Figs. 15-4 and 15-5). This pattern, sparing the epicardial and endocardial layers, may or may not involve more than one segment of the myocardium. Some investigators have performed electron microscopy and viral cultures, and have demonstrated a pattern that may, by purely imaging techniques, identify the offending pathogen. If the linear stripe occurs in the mid anterior and anteroseptal segment, there is a high likelihood that the agent is human herpesvirus 6 (HHV-6), whereas if it occurs in the posterior lateral wall (see Fig. 15-6) the agent is likely to be a parvovirus. CMR appears to be able to aid further in the clinical translation of this diagnostic strategy, because there have been limited observations that the septal pattern has a more ominous prognosis in that there is more left ventricular dysfunction, both globally and segmentally, whereas the posterior pattern has a more benign presentation with less deleterious impact on segmental function. Finally, depending on the location of the pattern, a prediction of interim left ventricular function can be made in that the septal pattern demonstrates limited recovery at several months after infection, whereas
the posterior pattern generally correlates with considerable improvement in left ventricular function (see Fig. 15-7).
the posterior pattern generally correlates with considerable improvement in left ventricular function (see Fig. 15-7).
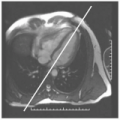
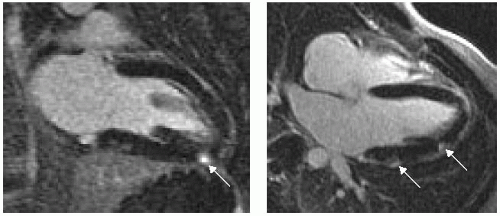 FIGURE 15-1 Delayed hyperenhancement (DHE) taken at 2 minutes after contrast injection, revealing focal high T1 signal of myocardium. Images relate to case study 1. |
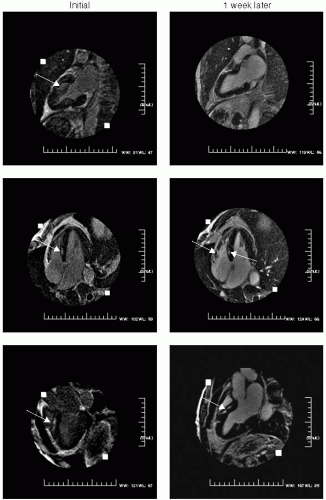 FIGURE 15-2 Delayed hyperenhancement (DHE) taken at 2 minutes after contrast injection, revealing focal high T1 signal of myocardium in the two-, four-, and three-chamber views taken 1 week apart. Images relate to case study 2. |
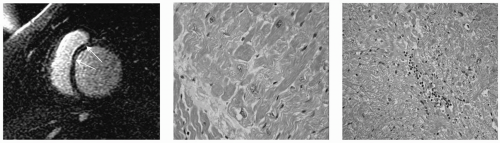
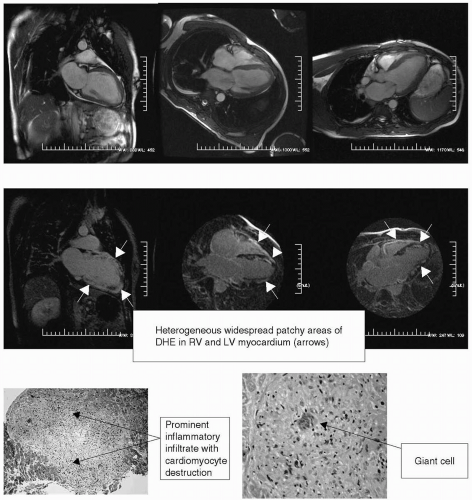 FIGURE 15-3 Steady state free precession (SSFP) sequences (top panel) and matching delayed hyperenhancement (DHE) images (lower panel). Histology slides (right panel) demonstrating evidence of granulomatous formation with a giant cell (arrow). Images relate to case study 3. |
Finally, in the absence of a CAD etiology, a finding of any one of the preceding patterns appears to be very helpful in explaining the etiology of the nonischemic cardiomyopathy. Upward of 40% of viral cardiomyopathies appear to have either a midwall stripe and/or a heterogeneous pattern, whereas 15% present with a bright pattern in the right ventricular septal insertion points. Therefore, when a nonischemic patient presents with a viral cardiomyopathy, there is a 50% chance of formally defining, or strongly indicating, the etiology. However, it should be noted that in fully 100% of this population, providing volumetric and left ventricular functional metrics is beneficial.
The finding of the midwall stripe has been proposed to be pathognomonic for a viral cardiomyopathy. However, we have recently seen several cases in which biopsy showed that the midwall stripe to be a forme fruste of idiopathic cardiomyopathy (see Fig. 15-4) and in another case a fatty pattern was seen. Early data supports that a finding of this pattern by itself may denote an adverse cardiovascular prognostic finding.
The finding of the midwall stripe has been proposed to be pathognomonic for a viral cardiomyopathy. However, we have recently seen several cases in which biopsy showed that the midwall stripe to be a forme fruste of idiopathic cardiomyopathy (see Fig. 15-4) and in another case a fatty pattern was seen. Early data supports that a finding of this pattern by itself may denote an adverse cardiovascular prognostic finding.
CASE STUDIES
Case Study 1.
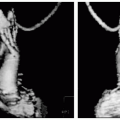
A 30-year-old man presents with sudden onset of chest pain (CP) that had grown from mild to extraordinarily painful over the preceding 6 hours. His electrocardiogram (ECG), echocardiogram, and a subsequent coronary catheterization were all normal. He underwent CMR for evaluation of an etiology for his presentation (see Fig. 15-1). No pericardial effusion was seen and left ventricular function was normal. However, on delayed hyperenhancement imaging several areas of epicardial enhancement are seen (arrows) consistent with myocarditis. Note the distinct absence of an endocardial or midwall pattern, which can distinguish between a MI and myocarditis.
Case Study 2.
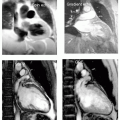
After a viral prodrome lasting 2 weeks, a healthy 32-year-old man presents with angst and growing chest discomfort for which he can find no relief. While not typical for coronary artery disease (CAD), given his strong family history, he undergoes an uneventful cardiac catheterization, which reveals normal coronary arteries. His echocardiogram is normal, and he is referred for cardiovascular magnetic resonance (CMR) which reveals several areas of mid and epicardial enhancement (see Fig. 15-2, left panel). The patient returned 1 week later for further evaluation of the acute versus chronic nature of his presentation, to aid prognosis (right panel). No distinct change was noted, preliminarily suggesting that this process resulted in damage to the myocardium.
Case Study 3.
40-year-old man with insidious onset of progressive shortness of breath (SOB) and fatigue over a 3-week period. CAD is ruled out by catheterization. While the author was in service we performed a CMR to assess functional and structural details of the heart (see Fig. 15-3). CMR showed severe LV systolic dysfunction with following functional data:
Left ventricular end-diastolic volume index (LVEDVI) = 101.1 mL/m2; left ventricular ejection fraction (LVEF) = 27.2%; right ventricular end-diastolic volume index (RVEDVI) = 86.6 mL/m2; right ventricular ejection fraction (RVEF) = 23.3%; left ventricular end-systolic volume index (LVESVI) = 73.7 mL/m2; left ventricular mass index (LVMI) = 78.8 g/m2; and right ventricular end-systolic volume index (RVESVI) = 20.2 mL/m2. CMR DHE images demonstrated widespread, extremely heterogeneous, dense and patchy areas of myocardial enhancement of left ventricles (LVs) and right ventricles (RVs). This is consistent with a severe inflammatory process. A diagnosis of giant cell myocarditis was made by CMR. One day later endomyocardial biopsy (EMB) confirmed the diagnosis of giant cell myocarditis.
Case Study 4.
An 18-year-old male adolescent with progressive SOB and fatigue over 4 days before presentation. CAD is ruled out by catheterization. The patient had a paternal cousin who had similar presentation when he was 23-year-old and received heart transplant. CMR was performed to assess functional and structural details of the heart. CMR data is as follows (see Fig. 15-4): LVEDVI = 175.9 mL/m2; LVEF = 4.7%; LVESVI = 167.5 mL/m2; and LVMI = 77.5 g/m2. CMR functional images demonstrated markedly dilated LV and RV. The LV myocardium was thinned. On DHE sequence there was a midwall septal stripe of enhancement (arrows). To date this is considered as a sign of viral myocarditis (most commonly HHV-6); therefore a preliminary diagnosis of viral myocarditis was made. The patient underwent percutaneous EMB which showed hypertrophy of myocytes but no myocyte destruction or cellular inflammation (middle panel). Viral cytopathic effects were not identified and staining did not show fibrosis or amyloid deposition. He underwent cardiac transplantation and the explanted heart tissue was examined by staining and electron microscopy, which again did not show signs of viral myocarditis (right panel). An inconclusive diagnosis of idiopathic dilated cardiomyopathy (DCM) was made based on the pathologic information. However, later his younger sister was screened by CMR for myocardial structure and function and was found to have biventricular dilatation with mild LV systolic dysfunction. On the basis of these findings, diagnosis of idiopathic DCM was reversed to familial DCM.
Case Study 5.
A 45-year-old man presents with signs and symptoms consistent with congestive heart failure, and is demonstrated to have a DCM by echocardiography. Delayed hyperenhancement sequences demonstrate a striking midwall stripe tracking through the anterior septum and inferior septal territories without any other regions of hyperenhancement, consistent with viral cardiomyopathy (see Fig. 15-5). This has recently been reported to be nearly pathognomonic with a viral cardiomyopathy, suggesting that this is the etiology for this patient’s presentation.
Case Study 6.
65-year-old white woman presents with SOB, fatigue, and left bundle branch block (LBBB) pattern; what is the etiology (see Fig. 15-6)? There is no evidence of a subendocardial hyperenhancement pattern to suggest a MI. A specific linear dense DHE pattern is seen in the basal anteroseptum. In approximately 40% of patients who are ultimately defined to have viral cardiomyopathy, a similar linear DHE pattern is present within the midwall. In early reports, this pattern denotes an adverse cardiovascular prognostic finding.
Case Study 7.
A 55-year-old man presents with a history of the viral prodrome that occurred approximately 1 year before his presentation for CMR (see Fig. 15-7). Delayed hyperenhancement imaging demonstrates a midwall stripe (arrows) supporting a diagnosis of a viral cardiomyopathy. Recent data suggest that the septal pattern, in addition to carrying a bad prognosis, may be due to the viral antigen HHV-6. This is in stark contrast to an infralateral or posterolateral pattern, which in many cases is of the antigen parvovirus, which carries a more favorable prognosis.
Case Study 8.
A 67-year-old black woman presents with 2 years of fatigue, SOB, and weight gain and peculiar findings on echocardiography in which there were biapical intermediate echoic signals. A CMR was performed (see Fig. 15-8) demonstrating severe biventricular dysfunction with moderate (2+) mitral and tricuspid regurgitation. Delayed hyperenhancement was performed, which demonstrates the following: (a) no evidence of a typical infarction pattern, (b) bilateral apical densities that fail to enhance with hyperenhancement imaging, (c) subtle, but important midwall interior post-contrast enhancement, as well as right ventricular septal insertion point hyperenhancement. This pattern is diagnostic for a viral cardiomyopathy with resultant severe biventricular dysfunction and biventricular apical thrombus formation assessed using only two CMR sequences.
Case Study 9.
Evaluation of cardiomyopathy; part of an integrated approach. Using a combination of SSFP functional images, first pass perfusion, and delayed hyperenhancement, a myriad of cardiac pathology can be identified, allowing the clinician to determine with a high probability the etiology of the patient who presents with cardiomyopathy (see Fig. 15-9). An integrated imaging approach that is rapid, efficient, noninvasive, with no morbidity or mortality has clear advantage over current standard approaches which incorporate traditionally invasive cardiac catheterization and radionuclide imaging with or without biopsy. Such an approach that is also inexpensive has obvious, clinical translation, as well as socioeconomic implications.
Case Study 10.
A routine cardiac CMR is performed in a middle-aged woman and an abnormality is seen in the very tip of the LV apex (see Fig. 15-10, arrows). Using SSFP images to demonstrate the contractile nature of the myocardium, with delayed hyperenhancement to demonstrate the lack of post-contrast enhancement, the abnormality is defined to be a benign LV diverticulum. No further therapy was needed. However, it is prudent to periodically follow this every one to 2 years to assure that no increase in size has occurred. Theoretically, if enlarged, this could be a favorable milieu for clot formation and therefore, warrant anticoagulation.
Case Study 11.
A 54-year-old white woman presents with dyspnea on exertion, early exercise fatigue, and a peculiar mass seen on an echocardiogram. CMR demonstrates a hyperdynamic LV with 3D EF of 80% and a moderately sized circular thin walled apical mass with flow in it (see Fig. 15-11). The limited differential diagnosis is LV aneurysm, LV pseudoaneurysm, or LV diverticulum, or something rare. She had no history of an MI, making the first unlikely, but either of the first two as occult findings could be possible. However, there is a narrow neck, pointing toward either a pseudoaneurysm or an LV diverticulum. The radio frequency (RF) tissue tagging (not shown) demonstrates no contraction to the apical region, discounting these as the diagnosis. The DHE image is used to evaluate for the presence of occult infarction, which is clearly demonstrated, and shown in lower right panel (arrows) as a very thin, well delineated, nearly transmural DHE signal. From this we concluded this was a psuedoaneurysm both by morphologic features of a narrow neck and by occult DHE. As the patient was extremely symptomatic, she underwent surgery for excision, under the premise that her hyperdynamic state was exaggerated (or caused) by this lesion, with high velocity “to and fro” flow seen in the apical mass. Surgical pathology however demonstrated that this was a hamartoma. Embryologically, a hamartoma is nearly identical to cardiac muscle. This is a case where DHE cannot differentiate cardiac muscle versus a muscle-like structure, leading to an erroneous, but logical CMR




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree