Fig. 1
Homemade 68Ga purification with cationic resin
2.5 67Ga/68Ga Purification: Isopropyl Ether Extraction System
2.5.1 Liquid–Liquid Solvent Extraction
The second method of purification was conventional solvent extraction using diisopropyl ether (Nachtrieb and Fryxell 1949; Brown 1971). 67Ga was diluted in 3.5 mL 7 mol L−1 HCl and extracted three times with 3.5 mL diisopropyl ether. The resulting solution, 10.5 mL diisopropyl ether containing 67Ga, was equilibrated three times with 10.0 mL deionized water, where 67Ga was back-extracted to the aqueous phase. To reduce the final volume containing radioactive gallium, the volume of extraction was reduced to 4.0 mL.
2.5.2 Column Chromatography Solvent Extraction
The purification studies shifted towards preparation of an extraction chromatography column based on absorption of diisopropyl ether in XAD-16. The resin was washed with ethanol and water once. The dried resin (1 g) was kept in contact with 1.4 mL diisopropyl ether during 24 h at 4°C.
The first column was a 10 mm glass column and the second column was a micropipette, as shown in Fig. 2. Furthermore, addition of TiCl3 and 1% NaI to reduce Fe(III) present in the solution to Fe(II) was also studied. TiCl3 can reduce Fe(III), however it is a contaminant for 68Ga labeling. Iodide can also reduce Fe(III), albeit less effectively than TiCl3. Fe(III) and Ga(III) have chemical similarity, which makes Fe(III) a competitor with Ga(III) in labeling procedures.
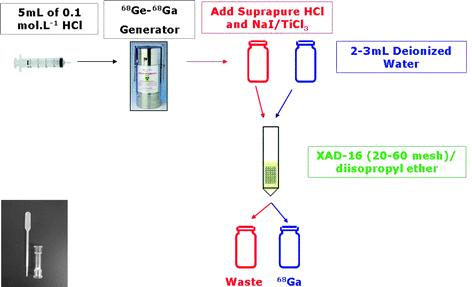

Fig. 2
Homemade 67Ga/68Ga purification with extraction chromatography column
3 Results and Discussion
3.1 68Ge/68Ga Generator Performance
Figure 3 shows the 68Ge/68Ga generator efficiency, averaging (71.2 ± 4.0)%. The 68Ge/68Ga generator was relatively new, having been calibrated in January 2011. It is essential to obtain good results for elution of the 68Ge/68Ga generator, mainly in case of manual elution, in terms of elution yield and time for the process.
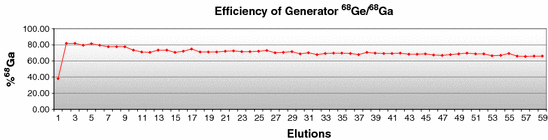

Fig. 3
68Ge/68Ga generator efficiency measured during 6 months
In addition, Fig. 4 shows the importance of often eluting the 68Ge/68Ga generator. Elution was performed every day, and the levels of impurities were reduced.
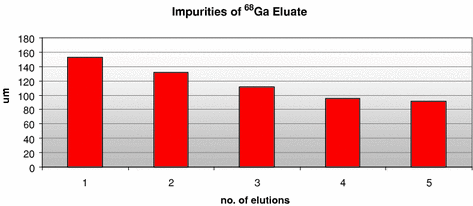

Fig. 4
Level of impurities measured in the eluate, performed five times consecutively
3.2 Water Purification
The results of analyses showed that the main contaminants, Fe and Zn, were reduced after Chelex 100® purification, by (82 ± 5)% and (91 ± 10)% (n = 3), respectively. Other analyzed metals, such as Cu, Ge, Ti, and Ni, were not significantly present in deionized water.
3.3 67Ga/68Ga Purification on Cation Exchange Resin
The first purification method was applied in a syringe used as chromatography column with volume of 0.5 mL of AG50W-X8 (200–400 mesh). Although the results for reduction of metals (87.0 ± 3.6, n = 3) and 68Ga yield (70.9 ± 2.8, n = 3) were good, the processing time was 15 min and the final volume containing 68Ga was 1 mL acetone/12 mol L−1 HCl (97:0.4)%, which are both high considering the short half-life of 68Ga radioisotope and the importance of reducing the final volume in order to minimize contaminants and the mass of acetone and hydrochloric acid.
The chromatography column was changed for a smaller one; consequently, the resin volume was reduced to 0.05 mL. With this effort, processing time was reduced to 5 min and the purified 68Ga was recovered in 0.4 mL acetone/12 mol L−1 HCl (97:0.4)%. The reduction of metals was (97.0 ± 2.7)% (n = 3), however the 68Ga yield was decreased to (60.3 ± 5.7)% (n = 3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree