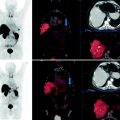
Fig. 1
Purification procedure, schematic drawing
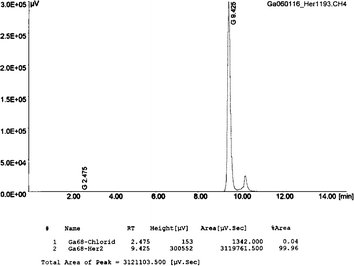
Fig. 2
HPLC of the final product. Column: RP-18, LiChroCART 250-4, LiChrospher 100, RP-18e (5 μm); solvent A: acetonitrile solution in water (5%), 0.1% TFA; solvent B: 95% acetonitrile solution in water, 0.1% TFA; flow rate: 1.2 mL/min; gradient: from 0–2 min 100% A, 3–15 min to 100% B. 68GaCl3: (R t = 2.5 min) 0.04%, 68Ga-Affibody: (R t = 9.4 min) 99.96%
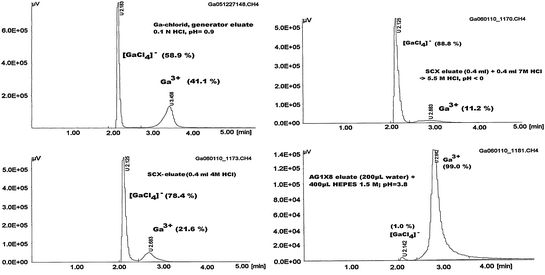
Fig. 3
HPLC of 68GaCl3 samples at different HCl concentrations. Column RP-18, LiChroCART 250-4, LiChrospher 100, RP-18e (5 μm); solvent A: acetonitrile solution in water (5%), 0.1% TFA; solvent B: 95% acetonitrile solution in water, 0.1% TFA; flow rate: 1.2 mL/min; gradient: from 0–2 min 100% A, 3–5 min to 30% B
2.4 Example: Synthesis of 68Ga-BPAMD
2.4.1 Introduction
For the 68Ga-labeled bisphosphonate monoamide derivative of DOTA (BPAMD) for patient studies, the combined cationic/anionic 68Ga eluent purification was successfully used in our department. The widely used cationic labeling procedure (Zhernosekov et al. 2007) leads to a nonphysiologic final product with pH lower than 2. Neutralization of the final product with sodium hydrogencarbonate (8.4%) is not possible, because of the decomposition of 68Ga-BPAMD. Furthermore, the final product contains nonreacted and free 68Ga3+ in different concentrations, so that a purification step is necessary. The combined purification method avoids these problems.
2.4.2 Labeling Procedure
The 68Ga generator was eluted with a total of 10 mL 0.1 M HCl, and the 68Ga was collected on a SCX cartridge (Varian, Bond Elut-SCX, 100 mg, 1 mL). This cartridge was then eluted with 5.5 M HCl directly through an anion exchanger cartridge.
For this anionic purification step we used a combined SAX cartridge (Alltech SAX Extract Clean SAX, 100 mg/1.5 mL), covered with 70 mg AG1X8 (BIO-RAD, 200–400 Mesh, hydroxide form), preconditioned with 1 mL 5.5 M HCl. The cartridge was dried with a stream of inert gas or air for 1 min, and 68Ga was then eluted with 1 mL water followed by elution with 0.5 mL aqueous 1.5 M ammonium acetate solution into the reaction vial with 1 mL water, 0.5 mL aqueous 1.5 M ammonium acetate solution, and 20 μg BPAMD. Then, the reaction mixture was heated to 100°C for 12 min.
2.4.3 Results
The 68Ga3+ was completely bonded, and after sterile filtration the radiochemical yield was about 55% (n.d.c.). This procedure leads to a final product with radiochemical purity greater than 95% without further purification steps. The pH of the final solution is about 4. Determination of other impurities by gas chromatography is not necessary. No organic solvents were added. The use of our developed combined SAX/AG1X8 cartridge increased the yield significantly. The instant thin layer chromatography (ITLC) quality control (Fig. 4) determines free 68Ga3+ as well as potentially formed 68Ga hydroxide.


Fig. 4
TLC of sterile filtered reaction solution synthesized by the combined cationic–anionic purification method (ITLC-SG; solvent: 0.1 M citric acid)
We thereby developed a reproducible and applicable labeling procedure for synthesis of 68Ga-BPAMD. The reaction delivers the product with radiochemical purity higher than 95%. Subsequent purification is not necessary, and the pH of the reaction solution is about 4.
3 A New Highly Efficient NaCl-Based Cationic 68Ge/68Ga Generator Eluate Purification: The Basis for Effective 68Ga Labeling
3.1 Aim
The aim is to develop efficient 68Ga labeling procedures for routine application in clinical practice. The purification procedure for the 68Ge/68Ga eluate should reduce handling with concentrated HCl, and should form the labeled final product in high yield with high purity. Use of acetone or other organic solvents or compounds such as HEPES should be avoided. Handling should be reduced to a minimum of simple steps and should allow transfer to an automated system.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree