Fig. 1
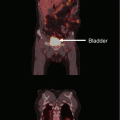
A 36-year-old female presented with early-stage, unfavorable Hodgkin lymphoma and was treated with combined modality therapy consisting of six cycles of ABVD followed by low-dose (20 Gy) consolidation RT (panel a). Her disease recurred in the mediastinum (panel b, red contour) within the RT field. She received three cycles of ICE followed by high-dose chemotherapy and autologous stem cell transplant. One year later, she was found to have recurrence at the same site in the mediastinum. She declined further chemotherapy. A definitive course of RT (40 Gy) was given (panel b, green contour). She remains without evidence of recurrence 4 years after completing salvage re-irradiation
Another circumstance is relapsed disease within the transplant setting. Investigators from Memorial Sloan Kettering Cancer Center reported on 65 patients with relapsed/refractory Hodgkin lymphoma, 60 % of whom had received prior radiation therapy (Moskowitz et al. 2001). The treatment program began with two cycles of ICE (ifosfamide, carboplatin, etoposide). If a satisfactory response was achieved, then accelerated fractionation, involved-field radiation therapy was pursued to patients with disease ≥5 cm at relapse or who had residual disease after ICE (1.8 Gy bid to 18–36 Gy, depending on disease status and prior radiation therapy). Upon completion of involved-field radiation therapy, patients proceeded with total lymphoid irradiation (1.8 Gy bid to 18 Gy). This was followed by further chemotherapy and autologous stem cell transplant. At a median follow-up of 43 months, the 5-year event-free survival was 58 %. Re-irradiation only to involved sites, without total lymphoid irradiation, may also be an appropriate strategy in select patients with relapsed disease after combined modality therapy. Low doses (~20 Gy) may be appropriate depending on the clinical circumstances.
Finally, radiation therapy can be utilized to palliate local symptoms in patients with refractory Hodgkin lymphoma. Durable responses can be achieved in the majority of patients with 20–30 Gy (Kaplan 1972). Such doses are rarely associated with significant side effects and can be safely given, using modern techniques, even when prior radiation therapy has been administered (Fig. 2).


Fig. 2
A 51-year-old male with refractory Hodgkin lymphoma, who had previously been treated with multiple courses of RT to the chest and abdomen, presented with paralysis due to epidural disease in the lower thoracic spine (white solid arrow, panel a). Due to prior RT to that area that approached spinal cord tolerance, he received 14 Gy with conventional RT which cleared the disease in the epidural space (white dashed arrow, panel b). Due to persistent disease in the surrounding bone and soft tissues, he received a stereotactic body radiation therapy boost with sparing of the spinal cord (black solid arrow, panel c) consisting of 12 Gy in 3 fractions (yellow contour, black dashed arrow, panel c). Thus, the total dose to gross disease in the spine was 26 Gy. Over the course of several months his lower extremity strength improved, he became ambulatory with the assistance of a walker and returned to work
3 Diffuse Large B-cell Lymphoma
As with Hodgkin lymphoma, early-stage (I-II) diffuse large B-cell lymphoma is often managed with a combination of systemic therapy and radiation therapy. This typically consists of three to six cycles of immunochemotherapy, most commonly R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), followed by consolidation radiation therapy. The Eastern Cooperative Oncology Group study 1484 demonstrated that consolidation radiation therapy after eight cycles of CHOP was associated with reduced rates of local failure (4 % vs. 16 %, p = 0.06) and improved 6-year disease-free survival (73 % vs. 56 %, p = 0.05), the primary endpoint of the study (Horning et al. 2004). A dose of 30 Gy is typically utilized after a complete response to immunochemotherapy is achieved. Higher doses (≥40 Gy) may be necessary in the setting of a partial response or with refractory disease.
Patients with advanced disease (III-IV) are generally treated with immunochemotherapy alone. Radiation therapy is utilized in select patients. Indications for radiation therapy include bulky disease (Held et al. 2014), partial response to systemic therapy (Dorth et al. 2011; Sehn et al. 2013), and limited skeletal involvement (Held et al. 2013). Select patients with technically advanced disease but limited presentations may also benefit from consolidation radiation therapy. In advanced stages, as more chemotherapy cycles are generally used with more widespread disease (and thus larger field sizes), lower doses such as 20 Gy seem reasonable (Dorth et al. 2012).
About a third of patients with diffuse large B-cell lymphoma will suffer a relapse. First-line treatment at relapse for appropriate candidates is non-cross-reactive chemotherapy followed by high-dose chemotherapy and autologous hematopoietic cell transplantation. Further radiation therapy must be tailored to the individual circumstances. Factors that must be considered include the prior dose of radiation utilized, field treated, interval between initial treatment and relapse, extent of relapse, response to salvage chemotherapy, etc.
Total body irradiation may be utilized, in select circumstances, in the relapsed setting as part of the conditioning regimen (Fig. 3). As doses of 12–14 Gy are required for transplant, this is usually feasible without significant risk. A second course of localized radiation therapy in the salvage setting may also be feasible depending on the circumstances. Finally, in those patients who are nonresponders to high-dose chemotherapy, fail transplant, or have a poor performance status, palliative radiation therapy can be considered. Radiation can be used in this setting to relieve symptomatic areas. As in Hodgkin lymphoma, re-treatment is generally possible due to the lower consolidation radiation doses needed for lymphoma. Response rates have been reported to be as high as 50–80 % with doses as low as 4 Gy (Murthy et al. 2008; Haas et al. 2005). Such doses can be utilized in almost all patients. If this is unsuccessful, more conventional palliative doses can be pursued (20–24 Gy).


Fig. 3
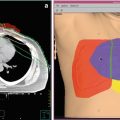
A 52-year-old male presented with low back pain and found to have a retroperitoneal mass (white arrow, panel a). Biopsy showed DLBCL, and positron emission tomography-computed tomography (PET-CT) imaging showed diffuse adenopathy with bulky retroperitoneal disease invading adjacent vertebral bodies and obstructing the left ureter leading to kidney dysfunction. He received six cycles of R-CHOP, achieving a complete response by PET-CT, followed by 30 Gy consolidation RT to the intra-abdominal disease (panel b). One year later, he was found to have diffuse disease progression confirmed with biopsy. He only achieved a partial response to three different salvage chemotherapy regimens. With refractory disease, a myeloablative allogeneic stem cell transplant was recommended utilizing a TBI-based regimen with custom shielding of the right kidney (white arrow, panel c)
4 Follicular Lymphoma
Approximately 20 % of patients with follicular lymphoma present with localized (stage I or contiguous stage II) disease. The optimal treatment for these patients is controversial as no randomized studies have compared radiation therapy alone (the traditional standard) with more modern approaches such as immunochemotherapy, with or without radiation therapy (Friedberg et al. 2012), or observation. Large database studies suggest that radiation therapy improves survival in this setting (Vargo et al. 2015; Pugh et al. 2010). With radiation therapy alone, approximately 50 % of patients have long-term disease control. When the disease does relapse, the predominant pattern of failure is distant (i.e., originally uninvolved lymph node sites).
The majority of patients with follicular lymphoma present with advanced (stage III–IV) disease. Systemic therapy, consisting of both immunotherapy (e.g., rituximab) and chemotherapy, is the foundation of follicular lymphoma management. Radiation therapy can be beneficial in a number of circumstances. For example, in settings where there is localized disease progression or poor response to systemic therapy, especially if symptoms are present that require palliation, radiation therapy may be appropriately employed. Depending upon the clinical circumstances, it is often prudent to treat with a low-dose approach (2 Gy × 2). A total dose of 4 Gy is almost always well tolerated, and response rates exceed 80–90 % (Haas et al. 2003; Russo et al. 2013).
Local control with conventional doses of radiation therapy is very high (24–30 Gy). The most common re-irradiation scenario is in patients receiving 2 Gy × 2 for palliation who either fail to respond or subsequently relapse in field. In this circumstance, it is almost always feasible to give a more protracted regimen (20–30 Gy) which invariably leads to the desired response (Fig. 4) or, in the instance of relapsing patients, to give another 4 Gy.


Fig. 4
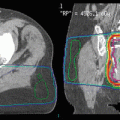
A 63-year-old female was diagnosed with stage III, low-grade, follicular lymphoma at age 53. She was initially treated with chemotherapy. Four years later, the disease recurred in the abdomen and she was treated with rituximab. Upon progression she received RT (4 Gy × 1) without response. The mass was re-biopsied and confirmed to be grade 2 follicular lymphoma. As she had a single site of active disease (8 cm), a more protracted course of RT was pursued (2 Gy qd to 30 Gy) (panel a, white arrow). She tolerated RT well and achieved a partial response at 1 year (panel b, white arrow)
5 Marginal Zone Lymphoma
The most common subtype of marginal zone lymphoma encountered by radiation oncologists is extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). These lymphomas most commonly arise within the stomach, orbital adnexa, parotid gland, skin, thyroid gland, and lung. The majority of patients present with localized disease. While an initial trial of antibiotics is appropriate for localized Helicobacter pylori-positive gastric MALT lymphoma, definitive radiation therapy is the preferred treatment for most other presentations. Complete responses to low-dose radiation therapy (24–30 Gy) are the norm (>95 %). Local failure is extraordinarily rare. In a recent large series from the Princess Margaret and Memorial Sloan Kettering Hospitals, failure within the radiation field occurred in 3–5 % of patients (Goda et al. 2010; Teckie et al. 2015). The dominant pattern of failure is at distant sites, typically in areas that are commonly involved by MALT. Thus, the need for radiation therapy for a previously irradiated area is unusual. In such circumstances, low-dose (2 Gy × 2) radiation could be used for palliative purposes (Russo et al. 2013). In select circumstances, depending upon initial dose and location, it may be possible to pursue a second definitive (~24–30 Gy) course of therapy. The more common circumstance is treating a new area for a localized distant relapse (Fig. 5).


Fig. 5
A 68-year-old male was diagnosed with Helicobacter pylori-negative gastric MALT lymphoma and achieved a durable complete remission with RT to 30 Gy (stomach in red, panel a). Four years later, he developed MALT lymphoma along the mandibular alveolar ridge. Most of the disease was removed at the time of biopsy. He received RT to 24 Gy (original extent of disease in red, panel b) and is without evidence of recurrence 3 years later
6 Plasma Cell Neoplasms
The most common plasma cell neoplasms that radiation oncologists encounter are solitary plasmacytomas and multiple myeloma. Only 5 % of plasma cell neoplasms are solitary lesions, which can develop at either osseous sites or in extramedullary locations, the latter most commonly in the head and neck region. For both, the preferred treatment is definitive radiation therapy to a dose of 40–45 Gy. Local control is largely determined by size of the primary tumor with larger tumors having a higher risk of local recurrence (Ozsahin et al. 2006; Tsang et al. 2001). The dominant pattern of failure is systemic progression to multiple myeloma. For osseous and extramedullary plasmacytomas, the 10-year risk of developing multiple myeloma is approximately 70 % and 35 %, respectively (Ozsahin et al. 2006). The majority of patients diagnosed with a plasma cell neoplasm have multiple myeloma. Painful lytic lesions are a common complication which a short course of radiation therapy can palliate. In general, doses of 8–24 Gy are recommended.
It is unusual for a solitary plasmacytoma to fail locally without evidence of systemic progression. In such cases, a second course of radiation therapy could be considered. There have been anecdotal reports of long-term disease control in such circumstances (Mendenhall et al. 1980). The location of the original disease and ability to avoid critical normal regional structures would dictate whether re-irradiation is feasible. Similarly, it is typically unnecessary to repeat a course of radiation therapy for myeloma. However, in circumstances where pain recurs and there is obvious radiographic or pathologic evidence of persistent disease, a second course of radiation is almost always feasible given the relatively low doses that are sufficient to palliate pain (Fig. 6).


Fig. 6
An 86-year-old female was treated for a painful left humeral lytic lesion with palliative RT (20 Gy in 4 Gy fractions) (panel a). Her pain improved but approximately 18 months later she developed a nondisplaced pathologic fracture of the proximal humeral metadiaphysis at the site of the original lytic lesion (panel b). She underwent open reduction and internal fixation with cement reconstruction (panel c). Biopsy confirmed myeloma. Due to persistent pain, she underwent a second course of RT using the same total dose but a higher dose per fraction (20 Gy in 5 Gy fractions) with subsequent improvement in pain (panel d)
7 CNS Lymphoma
Primary central nervous system (CNS) lymphoma is a relatively rare subtype of non-Hodgkin lymphoma. The most important treatment component is high-dose methotrexate, typically given in conjunction with other systemic agents including rituximab (Morris and Abrey 2009). The role of consolidation radiation therapy is controversial, primarily due to the risk of neurotoxicity in older adults after systemic high-dose methotrexate (Abrey et al. 1998). In the presence of a complete response, low-dose (23.4 Gy) whole brain radiation therapy is often utilized and seems to be associated with favorable clinical outcomes, including a low risk of subsequent failure in the brain and a low risk of neurotoxicity (Morris et al. 2013).
Secondary CNS lymphoma occurs when systemic disease secondarily involves the CNS. Current regimens in fit patients utilize a similar approach as primary CNS lymphoma, often in conjunction with high-dose chemotherapy and autologous stem cell transplant. The role of radiation therapy in secondary CNS lymphoma is not established.
Generally, repeat whole brain radiation therapy is not recommended in primary or secondary CNS lymphoma. Stereotactic radiosurgery, in which a tumor is treated with a single, high-dose conformal treatment, has been employed in the setting of limited intracranial progression after whole brain radiation therapy for CNS lymphomas (Kumar et al. 2015; Matsumoto et al. 2007; Kenai et al. 2006) (Fig. 7). Doses of 12–18 Gy have been utilized with overall response rates of ~85 %. Median survival, in heterogeneous populations of patients, is reported to be 10–17 months. As expected, radiosurgery is well tolerated without significant complications. However, distant brain failures are common after stereotactic radiosurgery (Matsumoto et al. 2007), presumably given the multifocality of intracranial disease.


Fig. 7
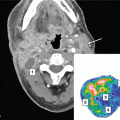
A 26-year-old male was treated for stage II DLBCL with eight cycles of R-CHOP but subsequently failed with two parenchymal lesions in the brain. He received further chemotherapy followed by an autologous stem cell transplant. As part of the preparative regimen, he received TBI (13.5 Gy) in addition to a boost to the brain (10 Gy). About 1 year later, he developed a new parenchymal brain lesion distant from his previous intracranial disease (panel a). Given the prior whole brain radiation therapy, this was treated with stereotactic radiosurgery (panel b, yellow contour is 15 Gy isodose line)
8 Cutaneous Lymphomas
Cutaneous lymphomas consist of both T-cell and B-cell histologies. The most common T-cell histologies are mycosis fungoides and CD30-positive lymphoproliferative disorders, which include lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. The B-cell histologies include primary cutaneous follicle center lymphoma, marginal zone lymphoma, and diffuse large B-cell lymphoma, leg type.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree