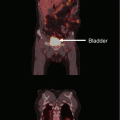
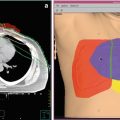
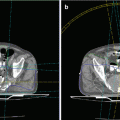
Fig. 1
Superficial hyperthermia of recurrent breast cancer
1.1.2 Interstitial and Endocavitary/Intraluminal Hyperthermia
Interstitial and endocavitary/intraluminal hyperthermia (IHT) methods may be used to treat tumors within or near body cavities, such as the head and neck area (Datta et al. 1990; Geiger et al. 2002; Strnad et al. 2015), the esophagus, or the anal canal (Kouloulias et al. 2005). Probes are placed inside the cavity or inserted into the tumor to deliver energy and heat the area directly. IHT is also often combined with interstitial brachytherapy (Fig. 2). Before and/or after high-dose-rate or pulsed-dose-rate afterloading irradiation, dedicated applicators (e.g., microwave antennas) are placed, e.g., within the brachytherapy tubes to additionally heat the target lesion. In clinical trials, additional IHT had been shown to improve overall survival and/or local recurrence rates of patients with bladder cancer (Colombo et al. 2003) as well as glioblastoma (Sneed et al. 1998

).

Fig. 2
Interstitial brachytherapy and hyperthermia of pre-irradiated recurrent tongue tumor
1.1.3 Deep Regional Hyperthermia
For locally advanced carcinomas of the abdomen, pelvis, and extremities, deep regional hyperthermia (RHT) is recommended. External applicators are positioned around the body cavity or organ to be treated, and microwave or radiofrequency radiation is focused on the area to raise its temperature (Fig. 3). The clinical efficacy of RHT has been proven in randomized trials, e.g., in locally advanced cervical carcinoma (van der Zee et al. 2000; Franckena et al. 2008), rectal cancer (Rau et al. 2002), and soft tissue sarcoma (Issels et al. 2010; Angele et al. 2014). Further indications for the additional use of RHT in combination with radiotherapy, chemotherapy, and chemoradiation are locally recurrent rectal (Juffermans et al. 2003), bladder (Ott et al. 2009; Wittlinger et al. 2009), prostate (Tilly et al. 2005), anal (Kouloulias et al. 2005), pancreatic (Schlemmer et al. 2004), and gastric cancer (Mochiki et al. 2007). Other regional hyperthermia approaches, for example, comprise perfusion techniques (Cornett et al. 2006) that can be used to treat cancers in the arms and legs, such as melanoma, or cancer in some organs, such as the liver or lung. In this procedure, some of the patient’s blood is removed, heated, and then perfused back into the limb or organ. Anticancer drugs are usually combined with this kind of hyperthermia treatment. Continuous hyperthermic peritoneal perfusion (HIPEC) is a technique used to treat disseminated cancers within the peritoneal cavity, including primary peritoneal mesothelioma and stomach cancer (Verwaal et al. 2008

).

Fig. 3
Deep regional hyperthermia of recurrent rectal cancer
1.1.4 Noninvasive Thermometry
The combination of deep regional hyperthermia systems with MRI systems has been established for clinical use in some major oncological departments in Europe during the past few years. Controlling the effect of hyperthermia during patient treatment is essential for quality-controlled therapy. Using the combination of a deep regional hyperthermia system with a dedicated MRI, the physician obtains detailed, three-dimensional information about temperature and perfusion during RHT (Gellermann et al. 2005 and 2006; Winter et al. 2015). After short MRI measuring sequences, color-coded temperature distribution images covering the whole region of interest are available for review and optimization of treatment parameters. With the introduction of noninvasive MRI thermometry techniques, RHT treatment quality is expected to further improve effective heating of tumors and avoidance of painful hot spots in the surrounding normal tissues.
1.1.5 Whole-Body Hyperthermia
Whole-body hyperthermia (WBHT) is usually used in the intention to treat systemically disseminated cancer. The body temperature is artificially raised to 41.5–42 °C, by the use of incubators or hot water blankets. Combined WBHT and chemotherapy was predominantly used in patients with incurable metastasized malignancies who developed progressive disease after first- or second-line systemic treatment. Some phase II trials proved the feasibility of WBHT with chemotherapy for metastasized rectal, prostate, and ovarian cancer, but an explicit benefit for the additional use of WBHT could not be shown because of the lack of randomized trials (Hildebrandt et al. 2005). In evidence-based medicine, WBHT currently receives limited attention.
1.2 Hyperthermia: Not an Alternative but Additive Treatment Option
In the perception of many cancer patients, hyperthermia is a treatment comparable to surgery and radio- and chemotherapy. Suffering from a malignant disease and confronted with radical therapeutic approaches in established evidence-based oncology scenarios, some of them are desperately searching for an alternative way to be cured. A heat treatment in the temperature range of 40–44 °C seems to be a quite attractive choice to avoid surgery and chemo- and/or radiotherapy. But, up to date, there is no data for the beneficial use of hyperthermia as sole modality in the treatment of any malignancy, neither for whole body nor local and regional hyperthermia techniques. This fact remains true despite of many contradictory advertisements in the Internet. The German Society of Radiation Oncology (DEGRO) published a guideline for the proper use of local and regional hyperthermia in the year 2000. It emphasizes that hyperthermia must be used exclusively in combination with radio- and/or chemotherapy for sensitization of the tumor tissue for the established anticancer treatment. Therefore, local and regional hyperthermia techniques are not alternative but additive options in the treatment of specific cancer diseases and have to fulfill quality criteria (Bruggmoser et al. 2012).
2 The Rationale to Integrate Hyperthermia into Multimodality Approaches for the Treatment of Recurrent Malignancies
The treatment of patients with locally recurrent malignant diseases is very often a difficult task due to heavy pretreatment including surgery, radiotherapy, and systemic therapies. In many cases, primary surgery for the recurrent tumor is not feasible because of the extent of disease (e.g., inflammatory chest wall recurrence of breast cancer after mastectomy) or infiltration of indispensable anatomic structures (e.g., substantial infiltration of the first sacral vertebra in case of recurrent rectal cancer or vessel infiltration in pancreatic cancer). Frequently, breast cancer patients already received the most effective chemotherapeutics like taxanes and anthracyclines during the treatment of the primary tumor, and a large number of patients with breast and rectal cancer have had adjuvant radiotherapy. All these preconditions have to be considered for an individualized treatment schedule of a locally relapsed tumor.
In case of previous irradiation doses of ≥50 Gy, many radiation oncologists prefer to prescribe a palliative dose of another 30 Gy to a non-resectable local recurrence of rectal cancer to avoid serious side effects, especially to the bladder and small intestine. However, it is well known that a pathologically confirmed complete resection of the local recurrence of a rectal cancer opens a curative perspective for up to 50 % of the patients (Dresen et al. 2008; Tanis et al. 2013). For these cases, the intensification of neoadjuvant treatment may lead to a higher rate of cure. Local and regional hyperthermia applications have been proven to be effective radio- and/or chemosensitizers in various tumor entities, usually with no significant contribution to late toxicity. In the treatment of locally recurrent breast cancer, one trial even found that the beneficial effects of additional hyperthermia were most pronounced in pre-irradiated patients (Vernon et al. 1996). This makes hyperthermia a very attractive partner for multimodality treatment approaches, especially for patients with recurrent disease and limited treatment options due to pretreatment.
3 Clinical Data
Whereas clinical data on the use and the effects of local and regional hyperthermia for the treatment of primary tumors is easy to find, data on the individualized treatment of locally recurrent tumors are sparse. The available data are summarized below.
3.1 Breast Cancer
A total of six randomized trials analyzed the effects of combined radiotherapy and LHT in breast cancer patients. Five of them were started between 1988 and 1991 by the UK Medical Research Council, the European Society of Hyperthermic Oncology (ESHO), the Dutch Hyperthermia Group, and the Princess Margaret Hospital/Ontario Cancer Institute (Vernon et al. 1996). Patients were eligible if they had locally advanced primary or recurrent breast cancer, and local radiotherapy was indicated in preference to surgery. The primary endpoint of all trials was local complete response. Slow recruitment led to a decision to collaborate and combine the trial results in one meta-analysis and report them in one publication. A total of 306 patients were analyzed: 44 % (135/306) received radiotherapy alone, and 56 % (171/306) received combined treatment. The biologically effective radiation doses ranged between 40 and 70 Gy, the single fraction doses between 1.8 and 4 Gy, and the overall treatment time between 2 and 5 weeks. In the five trials, heat was applied with different devices for superficial hyperthermia. Depending on the protocol, 2–8 hyperthermia treatments were scheduled during the radiation course. The duration of a single hyperthermia fraction ranged between 45 and 70 min and the target temperature aimed at 42.5–43 °C. The overall complete remission rate for radiotherapy alone was 41 % and for the combined treatment 59 % (p < 0.001). The most pronounced effect was observed in patients with recurrent lesions in previously irradiated areas, where further irradiation was limited to low doses. Of all patients who achieved a complete remission, 17 % of those having received combined treatment and 31 % of the radiotherapy only patients developed a local relapse during further follow-up (p = 0.007). The majority of the patients (227/306) showed progression of disease outside the treatment area during follow-up. The authors discussed this finding as the reason for the fact that overall survival was not improved by additional hyperthermia despite a significantly better local control. Hyperthermia was well tolerated and did not add to the clinically relevant acute or long-term toxicity compared to irradiation alone, even in those patients who had received prior radiotherapy. The authors concluded that the combined analysis of the five trials demonstrated the efficacy of hyperthermia as an adjunct to radiotherapy for the treatment of recurrent breast cancer.
The sixth randomized trial evaluating the role in the treatment of breast cancer was published in 2005 by Jones et al. of the Duke University, North Carolina (Jones et al. 2005). A total of 108 patients with superficially located tumors with different origins was analyzed in detail. The patients received either radiation alone (n = 52) or radiation combined with LHT (n = 56). Of the patients who received radiation alone, 63 % (33/52) had breast cancer or chest wall recurrences after a history of breast cancer, 12 % (6/52) had head and neck cancer, 12 % (6/52) had malignant melanoma, and 13 % (7/52) had other tumor histologies. In the combined group, the distribution was 66 % (37/56), 14 % (8/56), 9 % (5/56), and 11 % (6/56), respectively. A separate analysis for the breast cancer patients only was not performed. Among patients in both arms, the median radiation dose if prior radiation had been given was 41 Gy (range 18–66 Gy), and the median dose if no prior radiation had been given was 60 Gy (range, 24–70 Gy). Patients were randomly assigned to receive no further treatment (no-LHT arm) or additional hyperthermia (LHT arm) throughout the course of radiation, delivered twice a week for a maximum of 10 treatments, 1–2 h duration, separated by at least 48 h. Microwave spiral strip applicators, operating at 433 MHz, were used for external heating. Temperature was measured invasively. Maximally allowed temperatures in the adjacent normal tissue and tumor tissue were 43 °C and 50 °C, respectively. The complete remission rate in the LHT arm was 66 %; the CR rate in the no-HT arm was 42 % (p = 0.02). There was no significant difference in the proportion of patients in each arm who received additional systemic therapy. The improved local response in the LHT arm resulted in a significant difference in duration of local control between the two arms (p = 0.02). Local control at death or last follow-up was 48 % vs. 25 %, respectively. The improvement in complete response rate and local control was most pronounced for patients who were previously irradiated. Overall survival was not significantly different between the two groups. Recently published retrospective series concentrating exclusively on irresectable chest wall recurrence after breast cancer underline the value of additional superficial hyperthermia in combination with reirradiation for these patients (Oldenborg et al. 2015; Linthorst et al. 2013 and 2015).
3.2 Rectal Cancer
For patients with locally recurrent rectal cancer, there is no standardized treatment regimen, especially for the subgroup with a history of prior pelvic radiotherapy. The curative potential of surgery and radio- and chemotherapy as sole treatment option is very limited (Tanis et al. 2013). Therefore, a combined modality treatment approach is mandatory. Surgery as well as radiochemotherapy is established in the treatment of recurrent rectal cancer. But treatment results are still not satisfying. RHT has proved to be feasible in combination with radio- and/or chemotherapy (Juffermans et al. 2003; Schaffer et al. 2003; Milani et al. 2008). Two randomized trials for locally advanced primary rectal cancer showed the effectiveness of additional hyperthermia in increasing the response rate and prolonging the time to progression (Berdov and Menteshashvili 1990; Gani et al. 2016). Only few reports exist on combined modality treatment regimens including hyperthermia for recurrent rectal cancer. Endpoints of the following studies were feasibility and palliation.
Juffermans et al. (2003) evaluated the palliative effect of reirradiation and hyperthermia in patients with non-resectable, recurrent colorectal carcinoma in 54 patients. The total reirradiation dose varied from 24 to 32 Gy given in fractions of 4 Gy twice weekly. Three or four hyperthermia treatments were given once weekly during the radiation course. The combined treatment was feasible and well tolerated. Comparison of results from radiotherapy plus hyperthermia with results after radiotherapy alone suggested that additional hyperthermia prolonged the duration of palliation.
Schaffer et al. (2003) analyzed treatment and follow-up data of 14 patients with local recurrence of rectal cancer that were treated with radiotherapy, chemotherapy, and RHT. Nine patients had received previous irradiation and chemotherapy. These pretreated patients received a total irradiation dose of 30.6–39.6 Gy and 5-fluorouracil (5-FU) as a continuous infusion over 5 days per week (350 mg/m2/24 h) combined with RHT twice weekly. The 5 remaining not pretreated patients received irradiation of 45 Gy with an additional boost between 9 and 14.4 Gy, combined with continuous infusion of 5-FU on days 1–4, and 29–33 (500 mg/m2/24 h), and RHT twice a week. Among 13 evaluated cases, the overall objective response rate was 54 % (5 complete responses, 2 partial responses). At a mean follow-up of 13.9 months (range 5–32 months), 7 patients were alive. The therapeutic regimen appeared to be active in the treatment of local recurrences of rectal cancer.
Hildebrandt et al. (2004) reported on a pilot study of nine previously irradiated patients with local recurrence of rectal cancer treated with chemotherapy and hyperthermia. Hyperthermic chemotherapy was applied with weekly infusions of 43 mg/m2 of oxaliplatin (i.v., 120 min), 500 mg/m2 of folinic acid (i.v., 120 min), and 2.6 g/m2 of continuous infusion 5-FU (24 h) for 6 consecutive weeks. Oxaliplatin was started in parallel to pelvic RHT. A total of 67 applications were administered to nine patients and were well tolerated. A total of 55/67 (82 %) chemotherapy courses were applied without dose reduction. In 62/67 (93 %) hyperthermia sessions, a treatment time of more than 60 min was maintained. Eight out of 10 episodes of severe (WHO grade III) toxicity represented typical side effects of the chemotherapy given (nausea n = 4, diarrhea n = 3, neuropathy n = 1). Two severe adverse events were mainly attributable to hyperthermia (hematuria, n = 1; deterioration of a decubital ulcer, n = 1). No patient suffered disease progression according to WHO criteria during the treatment period. Two patients achieved a partial remission. It is concluded that hyperthermic chemotherapy with oxaliplatin, folinic acid, and 5-FU is feasible. Overall toxicity was moderate. Results, moreover, suggest a relevant palliative effect in patients with previously irradiated pelvic recurrence of rectal cancer.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree