Author
Case number
Technique/dose
Median survival
Brachytherapy
Scharfen et al. (1992)
66 GBM
Brachytherapy I-125 64.4 Gy
11.3 months
Sneed et al. (1997)
66 GBM
45 WHO III
Brachytherapy I-125 64.4 Gy
11.7 months
12.3 months
Gabayan et al. (2006)
81 GBM
14 WHO III
Gliasite brachytherapy 60 Gy at 10 mm
35.9 weeks
43.6 weeks
Tselis et al. (2007)
84 GBM
Brachytherapy Ir-192 40 Gy
37 weeks
Fabrini et al. (2009)
18 GBM
3 WHO III
HDR brachytherapy 18 Gy
8.0 months
Stereotactic radiosurgery
Shrieve et al. (1995)
86 GBM
Stereotactic radiosurgery 13 Gy
10.5 months
Cho et al. (1999)
46 GBM
Stereotactic radiosurgery 17 Gy
11.0 months
Combs et al. (2005a)
32 GBM
Stereotactic radiosurgery
Median 15 Gy (10–20 Gy)
10.0 months
Combs et al. (2005b)
54 GBM
39 WHO III
Stereotactic radiotherapy
36 Gy (15–62 Gy)
5 × 2 Gy conventional fractionation
8.0 months
16.0 months
Kong et al. (2008)
65 GBM
49 WHO III
Stereotactic radiosurgery 16 Gy
13.0 months
26.0 months
Patel et al. (2009)
36 GBM
Stereotactic radiosurgery 18 Gy
Fractionated stereotactic radiotherapy 36 Gy in 6 fractions
8.5 months
7.4 months
Cuneo et al. (2012)
49 GBM
Stereotactic radiosurgery 15 Gy
10 months
Hypofractionated stereotactic radiotherapy
Shepherd et al. (1997)
33 GBM
Hypofractionated conformal radiotherapy
Escalation 20–50 Gy
11.0 months
Hudes et al. (1999)
19 GBM
1 WHO III
Stereotactic hypofractionated radiotherapy
Escalation 24 Gy (3 Gy/F) – 35 Gy (3.5 Gy/F)
10.5 months
Lederman et al. (2000)
88 GBM
Stereotactic hypofractionated radiotherapy
Median 24 Gy in 4 fractions
7.0 months
Grosu et al. (2005)
44 GBM
Stereotactic hypofractionated radiotherapy
36 PET/SPECT 30 Gy
8 CT/MRI (6 × 5 Gy)
9.0 months
5.0 months
Fokas et al. (2009)
53 GBM
Stereotactic hypofractionated radiotherapy
30 Gy in 10 fractions
9.0 months
Fogh et al. (2010)
105 GBM
42 WHO III
Stereotactic hypofractionated radiotherapy
Median 35 Gy in 10 fractions
11.0 months
10.0 months
Palmer et al. (2015)
161 GBM
59 WHO III
Stereotactic hypofractionated radiotherapy
Median 35 Gy in 10 fractions
10.8 months
Conventionally fractionated stereotactic radiotherapy
Arcicasa et al. (1999)
31 GBM
Fractionated conventional 2D radiotherapy
34.5 Gy in 23 fractions (1.5 Gy/F)
13.7 months
Cho et al. (1999)
25 GBM
Conventional fractionated radiotherapy 37.5 Gy in 15 fractions
12.0 months
Koshi et al. (2007)
11 GBM
14 WHO III
Stereotactic radiotherapy
22 Gy in 8 fractions/8 F (+ hyperbaric oxygen)
11.0 months
19.0 months
Combs et al. (2008)
8 GBM
10 WHO III
7 WHO II
Stereotactic radiotherapy
36 Gy in 2 Gy per fraction (+ temozolomide 50 mg/m2)
9.0 months
5.1 Conformal Radiotherapy
Over the past decade, there have been improvements in radiotherapy and imaging techniques to improve target definition. 3D conventional radiotherapy using co-registered magnetic resonance imaging (MRI) has improved target definition and allows the dose to normal structures to be reduced. Further developments such as IMRT (intensity-modulated radiotherapy) can improve this further by improving conformality at the target using multiple modulated beams. With re-irradiation planning and treatment, the intention is generally to treat the area of recurrence with an adequate margin while avoiding the total dose to critical organs at risk within the CNS, such as the optic nerves or the brainstem, making highly conformal approaches very appealing in this context (Figs. 1 and 2).



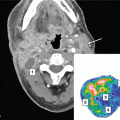
Fig. 1
CT and MRI-fused planning images for a patient treated for a recurrent glioma. Ten years previously treated with 55 Gy in 30 fractions over 6 weeks. Re-irradiated with 30 Gy in 6 fractions over 2 weeks. CTV (Blue contour) contrast-enhancing volume on T1-weighted MRI, PTV (Red contour) CTV + 0.5 cm margin

Fig. 2
Image showing four-field conformal radiotherapy axial plan for the same patient
5.2 Stereotactic Radiosurgery/Fractionated Stereotactic Radiotherapy
In brain radiotherapy, stereotactic methods offer optimal precision of target definition while minimising dose to the surrounding tissues. This treatment technique usually utilises exact positioning of the patient using a frame-based structure for three-dimensional localisation.
Stereotactic radiosurgery (delivered using the Leksell Gamma Knife, adapted linear accelerators, CyberKnife and other devices) is a non-invasive, highly conformal radiotherapy technique. It allows very accurate dose delivery with the patient in a fixed head frame, and multiple beam sources produce a steep dose gradient at the edge of the target, therefore allowing a highly precise dose to be delivered to tumour while sparing the surrounding normal tissues and organs at risk. The treatment is limited to smaller volumes as the risk of radiotherapy-related side effects increases with both volume treated and dose. The treatment is usually given in a single fraction (Figs. 3 and 4).



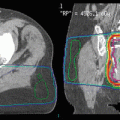
Fig. 3
A 57-year-old patient with recurrent glioblastoma of the right temporal lobe anterior to the surgical resection cavity. The targeted volume was 12.3 ml. The patient was treated with SRS to a dose of 18 Gy prescribed to the 90 % isodose line

Fig. 4
A 44-year-old patient with recurrent glioblastoma of the right temporal lobe. The targeted volume was 123.3 ml. The patient was treated with FSRT to a dose of 36 Gy in 6 fractions prescribed to the 90 % isodose line due to the large tumour volume and close proximity of critical structures
In fractionated stereotactic radiotherapy, the dose is divided over several fractions, which is made possible by using relocatable head frames or image-guided frameless systems. This has the radiobiological advantage of sparing toxicity to normal tissues due to fractionation and the allowance of normal tissue repair during treatment. This technique can therefore be used to treat larger volumes compared to stereotactic radiosurgery (Fig. 5).
Shepherd et al. (1997) reported on 29 recurrent high-grade glioma patients treated with a variety of doses of stereotactic hypofractionated re-irradiation who had a median survival of 11 months. This compared favourably to a matched cohort of patients treated with nitrosourea chemotherapy with a median survival of 7 months. In this study, a stereotactic radiotherapy dose of >40 Gy was found to be a significant predictor of radiation damage. There was also a trend towards higher risk of complications for larger volumes irradiated.
More recent data on response to radiosurgery or fractionated stereotactic radiotherapy in the retreatment of high-grade glioma reported a median survival of 8 months with radiological response rate of 40 % based on MRI criteria (Patel et al. 2009).
There is an ongoing clinical trial at the National Cancer Institute addressing hypofractionated stereotactic radiation therapy dose escalation (NCT02709226). The study is a phase I 3+3 design trial with three pre-planned dose levels: 3.5 Gy × 10 fractions, 3.5 Gy × 12 fractions and 3.5 Gy × 14 fractions.


Fig. 5
MRI images used for radiation treatment planning. The left panel depicts a conformal radiation treatment plan with large portions of the brain receiving at least 50 % of the prescription dose (blue isodose line). The right panel depicts a fractionated stereotactic radiation treatment plan with a much steeper dose gradient with less brain receiving at least 40 % of the prescription dose (blue isodose line)
5.3 Brachytherapy
Brachytherapy delivers radiation over a short distance therefore requiring the radiation source to be placed in close contact with the volume being treated. Most studies in the brain have used 125I or 192I. Placement of multiple sources of radiation around the surgical cavity is technically challenging to ensure an adequate and even dose distribution. A recent review by Combs et al. (2007) reports on the available but limited data on brachytherapy for recurrent gliomas (see Table 1). It should be noted that patients who are selected for brachytherapy are normally those with resectable tumours, good performance status and small volume of disease. High reoperation rates and radionecrosis incidence have been reported using these techniques.
5.4 Radiobiology of Re-irradiation of Gliomas
Although the biology of re-irradiation responses in the CNS remains to be fully understood, there is now a significant body of clinical and preclinical data which allow broad conclusions to be drawn and recommendations made. Mayer and Sminia (2008) identified and analysed 21 re-irradiation studies and reviewed the available clinical data on re-irradiation of gliomas with respect to tolerance of the normal brain tissue. They found that the incidence of toxicity, including radionecrosis, may be significantly under-reported since only symptomatic necrosis is likely to be recorded. According to their analysis, the major factor contributing to necrosis was the total dose received. There was no correlation between time to re-irradiation and the development of necrosis, although the minimum time interval between treatments in this data set was 3 months. Importantly, they concluded that the incidence of necrosis did not increase significantly until the total cumulative dose reached 100 Gy.
5.5 Conclusions Based on Clinical Evidence
Despite a large body of clinical work, there remains no standard protocol for re-irradiation of brain tumours. The clinical data are limited by the variety of techniques that have been used, which may be associated with different risks of toxicity. Specific toxicity is also often poorly reported in these studies and important variables are often not recorded.
Further limitations of the clinical evidence include problems distinguishing necrosis from recurrence on imaging. It is well established that standard, T1-weighted MRI sequences distinguish poorly between recurrent tumour and necrosis, which can often appear as a new enhancing lesion. The poor dose definitions also make comparisons between studies difficult. Some studies have used concomitant chemotherapy, which may have been a confounding factor. There are very few data on the quality of life following re-irradiation – an important consideration in a poor prognostic group (Nieder et al. 2008).
Overall, though, available data suggest that there may be a select group of patients with recurrent glioma in whom re-irradiation may be a safe and effective approach.
Ideally, radiotherapy should be highly conformal to keep the treated volume as small as possible and reduce late side effects to organs at risk and normal brain tissue. Many series suggest that limiting the target volume to approximately 4–5 cm minimises the risk of toxicity and suggests that if larger volumes are being targeted then consideration should be made for reducing the dose.
Most recurrent glioma patients will have received the equivalent of 55–60 Gy in 1.8–2 Gy per fraction when they were initially treated, and therefore not more than 40 Gy equivalent in a hypofractionated regime should be delivered when re-irradiating, aiming to keep the total dose less than 100 Gy (Mayer and Sminia 2008).
Available data do not suggest that there is an obvious limitation on the time between treatment courses, although most clinicians would not treat within a year, as these patients are likely to have primary treatment resistance.
Performance status of the patient and the potential impact on their quality of life should be taken into account. Consideration should be given to the impact of prolonged treatment courses in the context of poor prognosis disease.
6 Imaging
6.1 Target Definition
Contrast-enhanced thin-sliced MRI imaging using gadolinium remains the gold standard imaging modality for target delineation for gliomas. The problems are that following surgery or radiotherapy, the signal change based on gadolinium enhancement can be non-specific making accurate target definition a challenge. Most gliomas recur within 4 cm from the margin of the original lesion, and this is often where non-specific signal change due to surgery and prior treatment are also apparent (Gaspar et al. 1992; Chan et al. 2002).
To optimise the outcome of radiotherapy treatment, improved target definition is of paramount importance. In this context, alternative biological imaging may improve the definition of the relevant target, for example, amino acid PET (SPECT)/CT/MRI image fusion to determine the gross tumour volume is currently under investigation (GLIAA-NCT01252459).
Typically, the gross tumour volume is delineated as the new or progressive contrast-enhancing lesion. For stereotactic radiosurgery or fractionated stereotactic techniques, typically no margin is added; however, based on physician preference or other treatment parameters, a small 1–2 mm margin may be added. Recently, several studies have assessed the value of adding a lower dose to the surrounding T2/FLAIR volume due to the possibility that this may encompass low-grade or transforming tumour (Clark et al. 2014).
6.2 Diagnosing Radionecrosis
There is no gold standard imaging modality that can distinguish true tissue necrosis from tumour recurrence associated with recurrence (Ullrich et al. 2008). Conventional MRI techniques have limitations when discriminating tumour recurrence and treatment-induced injury. This may become possible with advances in imaging technology such as perfusion-weighted, diffusion-weighted and magnetic resonance spectroscopy (Bobek-Billewicz et al. 2010). Early studies using 18F-FDG PET reported sensitivities of 81–86 % and specificities of 40–94 % when distinguishing between radiation necrosis and recurrent tumour (Langleben and Segall 2000). Further studies are awaited but co-registration of MRI and amino acid tracers may improve the diagnostic accuracy (Chen 2007; Götz and Grosu 2013).
6.3 Diagnosing Progression
For many years, the Macdonald criteria developed in 1990 was the gold standard in assessing response to treatment in high-grade gliomas. These criteria were based on two-dimensional tumour measurements and clinical assessments (Macdonald et al. 1990). These criteria focused on the contrast-enhancing lesions only and have limited use to differentiate pseudoprogression from true progression. The new standardised response criteria for glioma clinical trials are the updated Response Assessment Criteria in Neuro-Oncology (RANO) criteria developed in 2010 (Wen et al. 2010). These updated criteria differentiate between measurable and non-measurable lesions on contrast-enhanced CT, MRI T1 post-contrast and T2 imaging. A measurable lesion is defined as a contrast-enhancing lesion with clearly demarcated margins, seen on two or more axial slices with a maximum diameter of at least 10 mm excluding a cystic cavity. Non-measurable lesions are those with cystic or necrotic components or a surgical cavity. For these lesions, the peripheral nodular component is considered measurable. Criteria for progression of disease based on RANO criteria include a 25 % or more increase in enhancing lesions despite increasing steroid dose; a significant increase in non-enhancing T2/FLAIR, not attributable to other causes; any new lesions; and clinical deterioration.
The diagnosis of true tumour progression can be difficult as approximately 20–30 % of patients may develop pseudoprogression after concurrent chemoradiotherapy (Brandsma et al. 2008). Pseudoprogression is a manifestation of treatment-related effects and is more likely in MGMT-methylated glioblastoma patients. Newer imaging techniques have been developed which may also aid in the differentiation between early tumour progression and pseudoprogression. Perfusion MRI imaging measuring cerebral blood volume changes may predict early tumour progression as true tumour progression manifests as an increase in relative cerebral blood volume (rCBV), and radionecrosis more likely manifests with a relative decrease in rCBV (Surapaneni et al. 2015). Additionally, diffusion-weighted MRI imaging and apparent diffusion coefficient maps may also predict early tumour progression as true progression is more likely correlated with low ADC values (Chu et al. 2013).
7 Combination Treatment
Few studies have addressed the addition of conventional cytotoxics when re-irradiating brain tumours. Some of the studies combining new agents with radiotherapy are described below. The combination of temozolomide and re-irradiation has been found to be safe and effective. In a study combining fractionated stereotactic radiotherapy and concomitant temozolomide in 25 patients with recurrent gliomas, median survival from re-irradiation was 8 months. Treatment was completed in all patients as scheduled without interruptions greater than 3 days, and no severe treatment-related side effects were observed (Combs et al. 2008). In a phase II trial, concurrent temozolomide delivered with conventional radiotherapy achieved an overall response rate of 20.6 % with a median progression-free survival of 10.1 months. The combined therapy was well tolerated and HR-QOL was improved (Osman 2014).
Darakchiev et al. (2008) reported on 34 patients with recurrent GBM and following re-resection who were treated by implantation with 125I seeds and Gliadel wafers to the resection bed. They documented that patients with a Karnofsky performance status less than 70 were more likely to have a worse outcome. One-year survival was 66 % but this was a small, non-randomised study, and brain necrosis was observed in 24 % of cases associated with a tumour volume of >30 cm3.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree