Study group
Classification
Definitions
Mayo Clinic (Suzuki et al. 1996)
Symptoms
S0
Asymptomatic
S1
Symptomatic without pain
S2
Symptomatic with pain
Degree and site of fixation
F0
No fixation
F1
Fixation to 1 point
F2
Fixation to 2 points
F3
Fixation to > 2 points
Yamada et al. (2001)
Pattern of pelvic fixation
Localized
Invasion to the adjacent pelvic organs or tissue
Sacral invasive
Invasion to lower sacrum (S3, S4, S5), coccyx, periosteum
Lateral invasive
Invasion to sciatic nerve, greater sciatic foramen, lateral pelvic wall, upper sacrum (S1, S2)
Wanebo et al. (1999)
Five stages
TR1
Limited invasion of the muscularis
TR2
Full-thickness penetration of the muscularispropria
TR3
Anastomotic recurrences with full-thickness penetration beyond the bowel wall and into the perirectal soft tissue
TR4
Invasion into adjacent organs without fixation
TR5
Invasion of the bony ligamentous pelvic including sacrum, pelvic/sidewalls or sacrotuberous/ischial ligaments
Memorial Sloan-Kettering (Moore et al. 2004)
Anatomical region involved
Axial
Anastomotic, mesorectal, perirectal soft tissue, perineum (APER)
Anterior
Genitourinary tract
Posterior
Sacrum and presacral fascia
Lateral
Soft tissues of the pelvic sidewall and the lateral bony pelvis
Leeds
Anatomical region involved
Central
Tumour confined to pelvic organs or connective tissue without contact with or invasion into the bone
Sacral
Tumour present in the presacral space and abuts on to or invades into sacrum
Sidewall
Tumour involving the structures on lateral pelvic sidewall, including greater sciatic foramen and sciatic nerve through to piriformis and gluteal region
Composite
Sacral and sidewall recurrence combined
Royal Marsden Hospital (Georgiou et al. 2013)
MRI; planes of dissection
C
Rectum or neorectum, intraluminal recurrence, perirectal fat or mesorectum, extraluminal recurrence
PR
Rectovesical pouch or rectouterine pouch of Douglas
AA PR
Ureters and iliac vessels above the peritoneal reflection, sigmoid colon, small bowel and lateral sidewall fascia
AB PR
Genitourinary system
L
Ureters, external and internal iliac vessels, lateral pelvic lymph nodes, sciatic nerve, sciatic notch, S1 and S2 nerve roots, piriformis or obturator internus muscle
P
Coccyx, presacral fascia, retrosacral space, sacrum up to the upper level of S1
I
Levator ani muscles, external sphincter complex, perineal scar (APER), ischioanal fossa
In a recent prospective study by the Royal Marsden Hospital, a new classification is described based on the extent of tumour invasion in each of seven intrapelvic compartments, as seen on preoperative pelvic MR imaging (Georgiou et al. 2013). These correspond to the fascial boundaries and planes of dissection between the pelvic organs, and are described as central (C), posterior (P), inferior (I), anterior above (AA) and anterior below (AB) the peritoneal reflection and lateral (L) and peritoneal reflection (PR). Such a MR-based classification system is particularly promising, since it allows for better understanding of tumour invasion within the pelvis, hence contributing to optimal surgical planning.
3 The Role of Surgery in LRRC
Resectability in recurrent rectal cancer can be defined as the ability to complete a surgical resection with a microscopically clear margin (R0) and acceptable postoperative morbidity and mortality. According to the recent consensus statement by the Beyond TME Collaborative Group (2013), absolute contraindications to resectability include bilateral sciatic nerve involvement and circumferential bone involvement. Benefits of surgery are unclear when the tumour extends through the sciatic notch, or encases the external iliac vessels or involves the sacrum above the S2/3 junction or irresectable distant metastases are present.
There are three broad pelvic site patterns that determine resectability: (i) central recurrence, (ii) sacral recurrence and (iii) lateral recurrence. For central recurrences, if the recurrence does not involve any of the anterior genitourinary structures, an abdominoperineal resection (APR) of the anus and neorectum is occasionally possible. Where there is involvement of the anterior urogenital structures, an extended multivisceral resection is required to achieve a R0 resection. When posterior structures are involved, more extended radical resections are often necessary. Where bony invasion is present, an R0 resection is only possible with a sacral resection. Recurrence involving the lateral pelvic sidewall is associated with the poorest chance of achieving an R0 resection (Moore et al. 2004). Wound healing is frequently impaired after previous chemoradiotherapy, and in selected patients, optimal healing is best achieved using a variety of pedicled flaps.
A summary of outcomes of exenterative surgery for LRRC from contemporary studies has been recently provided by Renehan (2016). R0 resection rates are about 50 % (range 38–62 %) (Ferenschild et al. 2009; Bhangu et al. 2014; Nielsen et al. 2012) with almost half of patients requiring sacrectomy. Less than half of patients remain free from disease at long term, with reported 3-year disease-free survival of 22 % (Nielsen et al. 2012) and 50 % (Bhangu et al. 2014) in two different series.
The morbidity and mortality rate can be as high as 60 % (range 25–60 %) and 8 % (range 0–8 %), respectively.
The complication rate might be higher in patients who undergo multivisceral resection versus those having single organ resection (Gezen et al. 2012).
Morbidity and mortality rate might be higher for more extended surgical procedures. For example, sacrectomy (compared with other operations) was associated with significantly higher mean blood loss, longer duration of surgery and longer length of stay. Cystectomy (compared with no cystectomy) was associated with longer duration of surgery. Perineal flap reconstruction (compared with primary closure or nonflap reconstruction) was associated with a longer mean operating time and a longer mean length of stay (Bhangu et al. 2014).
Unfortunately, only one-third to one-half of LRRCs will be resectable with conventional surgical procedures; the rest will require extended radical resection with removal of surrounding organs, to achieve clean margins. Optimizing patients before multivisceral resection is vital to minimize perioperative morbidity, requires a multispecialist approach and may best be achieved by formal cardiopulmonary testing (Beyond TME Collaborative 2013).
4 The Role of Reirradiation in LRRC
Neoadjuvant external beam radiation up to a dose of 50.4 Gy with concurrent chemotherapy is the standard of care for patients with LRRC who have not received any radiotherapy, since it has been demonstrated to improve the local control (Vermaas et al. 2005; Dresen et al. 2008). Indeed neoadjuvant long-course chemoradiotherapy has the potential to increase the resectability rate of delayed surgery in LRRC from 29.2 to 64.9 % (Dresen et al. 2008) and, by downstaging the tumour, may theoretically allow for less-extended surgical operations. In general, recurrent rectal cancers are treatment-resistant tumours. In a recent study at the Royal Marsden Hospital, only 9 % of patients with LRRC had a pathologic complete response compared to 17 % of patients with locally advanced primary tumour after long-course chemoradiation (Yu et al. 2014). Recurrent rectal cancer after previous irradiation might be even more radio resistant due to the possible origin from radio-resistant clones. Reirradiation is also challenging, because the surrounding normal tissues may have already received doses near the organ- or endpoint-specific tolerance dose during the primary treatment. Therefore reirradiation has been generally discouraged for the fear of prohibitive normal tissue complications, particularly of the small intestine and bladder. However, there is increasing evidence in clinical studies that reirradiation is tolerable and yields good results. Particularly in a recently published study including a large number of patients with LRRC, reirradiated patients had almost the same R0 resection rate and long-term local disease control as those who received full-course irradiation for the first time. Furthermore, despite more extensive surgical procedures in the reirradiation group, reflecting more advanced disease, no significant difference was noted in the rate of complications between the two treatment groups (Bosman et al. 2014).
Intraoperative radiation therapy might also represent a useful technique for these patients, with the possibility of precisely delivering a large single dose (10–20 Gy) to the surgically defined recurrence site and avoiding the surrounding normal tissues (Gunderson et al. 1996; Mannaerts et al. 2001).
A recent systematic review suggests that only 40 % of unselected consecutive patients with locally recurrent rectal cancer are candidates for intentionally curative treatment (Tanis et al. 2013). Surgical resection should be evaluated in all instances of isolated LRRC. However, in some cases, a resection is not possible, or medical reasons such as concomitant illnesses restrain the surgeon from surgical interventions. In other cases, surgery is performed, but a gross resection is not possible, and macroscopic tumour remains, which requires adjuvant treatment. The remaining patients with isolated LRRC might benefit from image-guided stereotactic radiotherapy, brachytherapy or particle beam radiotherapy with the aim of achieving both palliation and long-term local control (Combs et al. 2012).
4.1 Long-Course Reirradiation
The effects of conventional external beam reirradiation in terms of feasibility, toxicity and long-term outcomes in previously irradiated LRRCs were the subject of a recent systematic review (Guren et al. 2014) which included ten publications describing seven patient cohorts/studies for a total of 375 patients (range 13–103) (Mohiuddin et al. 1993, 1997, 2002; Lingareddy et al. 1997; Valentini et al. 1999, 2006; Das et al. 2010; Koom et al. 2012; Sun et al. 2012; Ng et al. 2013). Most studies were retrospectively designed, with highly variable therapies, patient populations and duration of follow-up. Median time since previous RT in different series ranged between 8 and 30 months and was longer than 24 months in most series. Reirradiation for rectal cancer was mostly given with hyperfractionated chemoradiotherapy to total doses of 30–40 Gy, although higher doses have been explored (range 23.4–50.2 Gy). EQD2Gy (α/β = 3 Gy) of previous irradiation ranged between 43.2 and 51.8 Gy3 with an estimated cumulative EQD2Gy with retreatment ranging between 71.9 and 101.7 Gy3 (Guren et al. 2014).
In older series, reirradiation was generally given by opposed lateral or three fields with a shrinking field technique, encompassing the presacral region or posterior pelvis (as prophylaxis for subclinical disease) and the gross tumour volume plus a margin of 1–4 cm (usually 2 cm) followed by a boost to the gross tumour volume (plus margin) only (Mohiuddin et al. 1993, 1997, 2002; Lingareddy et al. 1997; Valentini et al. 1999). In newer studies, reirradiation was delivered by multiple fields with a three-dimensional conformal or intensity-modulated technique, and the treatment volumes encompassed the gross tumour only with a 2-cm GTV to PTV margin (Valentini et al. 2006; Das et al. 2010; Koom et al. 2012; Sun et al. 2012; Ng et al. 2013).
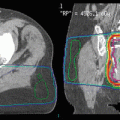
Figure 1 shows the possibility of small bowel sparing with intensity-modulated techniques.
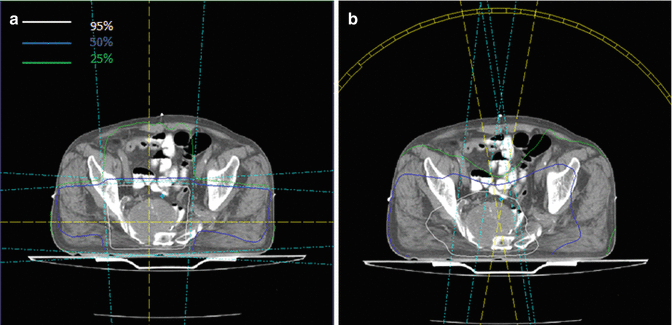

Fig. 1
Dose distribution of three-dimensional conformal radiotherapy (a) and volumetric-modulated arc therapy (VMAT) (b) for presacral relapse in a previously irradiated rectal cancer patient, showing small bowel sparing with VMAT
Disease control and survival outcomes in contemporary clinical trials of reirradiation (Valentini et al. 2006; Das et al. 2010; Koom et al. 2012; Sun et al. 2012; Ng et al. 2013; Milani et al. 2008; Bosman et al. 2014) are reported in Table 2. The proportion of patients who underwent resection after reirradiation varies widely (range 20–100 %). Differences in resection rates can be mainly explained by the fact that patients with unresectable disease or intraoperatively detected distant disease were not excluded from the initial study population in some studies. Furthermore, as previously said, the lack of a standard classification of LRRC negatively affects the possibility of comparing results between different series. Although pathological complete responses were rarely described, R0 resection was obtained in more than 70 % of operated patients in almost all series (range 39–89 %).
Table 2
Disease control and survival outcome in contemporary clinical trials of long-course reirradiation
Author, publication year and study design (inclusion period) | Patient n° | Previous radiation dose Gy, median value (range) | Reirradiation median total dose (range)/fractionation | Technique | Concurrent chemotherapy | Tumour resection n (%) | Local control | Distant metastases-free survival | Median survival months | Overall survival |
|---|---|---|---|---|---|---|---|---|---|---|
Bosman et al. (2014), retrospective (1994–2013) | 135 | 15–55 Gy | 30.6/1.8 or 30/2 Gy + IOERT boost 10 (R0), 12.5 (R1) or 15 (R2) Gy at the 90 % isodose | 3-field or 3DCRT | Yes (86.7 %) | 135/135 (100 %) 75/135 (55.7 %) | 45.9 % (5-year) | 56.6 % (5-year) | – | 34.1 % (5-year) |
Ng et al. (2013), retrospective (1997–2008) | 56 | 50.4 Gy (21–64) | 39.6 (20–39.6)/1.8 Gy | 3DCRT or IMRT | 5-FU | 11/56 (20 %) R0:8/11 (72 %) | – | – | All, 19 | – |
Resected, 39 | ||||||||||
Unresected, 15 | ||||||||||
Sun et al. (2012), prospective (2004–2008) | 72 | <50 Gy (not reported) | 30–36/1.2 Gy bid; nonresectable: redraw GTV, total 51.6–56.4 Gy | 3DCRT | Capecitabine | 18/72 (25 %) R0:16/18 (88 %) | – | – | All, 32 | All, 45.1 % (3-year) |
Koom et al. (2012), retrospective (2000–2007) | 22 | 54 Gy (45–59.4 Gy) | 50.2 Gy (30–66)/1.8–3 Gy | 3DCRT or IMRT | Yes | 5/22 (23 %) | All, 32 % (2-year) | – | All, 21 | All, 50 % (2-year) |
Das et al. (2010), retrospective (2001–2005) | 50 | 47 Gy (25–70) | 39Gy (if retreatment interval ≥ 1 year) or 30 (if retreatment interval < 1 year)/1.5 Gy bid +/− IORT, 5–10 | 3-field | Capecitabine | 18/50 (36 %) R0:7/18 (38.8 %) | All, 33 % (3-year) | – | All, 26 | All, 39 % (3-year) |
Resected, 47 % | Resected, 60 | Resected, 66 % | ||||||||
Unresected, 21 % | Unresected, 16 | Unresected, 27 % | ||||||||
Milani et al. (2008), retrospective (2000–2005) | 24 | 50.4 Gy (38.0 − 59.4 Gy) | 39.6 Gy (30.0–45.0 Gy)/1.8 Gy | 3–5 field | 5-FU + hyperthermia | 0 % | – | – | – | – |
Valentini et al. (2006), prospective (1997–2001) | 59 | 50.4 (30–55) | 30 Gy (+boost 10.8 Gy)/1.2 Gy bid | 3DCRT | 5-FU | 30/59 (51 %) R0:21/30 (70 %) | All, 38.8 % (actuarial 5- year) Resected (R0), 69.0 % | 42 % (actuarial 5 years) | All, 42 | All, 39.3 % (5-year) |
Resected, 44 | Resected (R0), 65.0 % | |||||||||
Unresected, 14 | Unresected (or partial tumour removal), 22.3 % |
Median survival for all patients with LRRC ranged between 19 and 42 months. Unresected patients had median survival time of 14–16 months, whereas patients who underwent surgical removal of tumour had median survival of 39–60 months. Nearly one-half of patients, with resected LRRC treated with multimodality approach including reirradiation, achieved long-term control of pelvic disease, and up to 65 % of them had long-term (5-year) survival. Even in unresected patients, after preoperative long-course chemo-reirradiation, long-term control can be achieved in about 20 % of cases with a significant proportion of long-term (5-year) survivors (up to 22 %). About 50 % of patients developed distant metastases during follow-up (Valentini et al. 2006; Bosman et al. 2014).
Reirradiation is highly effective for palliation. Eighty-three to 94 % of reirradiated patients experienced partial pain relief, rectal bleeding completely resolved in 100 % of patients and rectal mass was palliated in more than 80 % of patients with a median duration of symptom relief of 9, 10 and 8 months, respectively, for each symptom (Guren et al. 2014).
Probably due to more conformal treatment and reduced volumes, there was a trend towards less acute and late toxicity in recent studies as compared to older trials (Guren et al. 2014). In modern series (Table 3), treatment break or termination due to toxicity infrequently occurred (less than 5 %). The most commonly observed grade 3–4 acute toxicities were diarrhoea (5–10 %) and skin reactions (5 %). The most frequently reported late toxicities were gastrointestinal and urinary complications such as small bowel obstruction or stricture in up to 14 % of patients, fistula, chronic diarrhoea, cystitis and impaired wound healing. In the series by Koom et al., a high rate (27 %) of ureter stricture was also reported.
Table 3
Toxicity in contemporary clinical trials of long-course reirradiation
Author, publication year, and study design (inclusion period) | Grade 3–4 acute toxicity (%) | Treatment break or termination (toxicity) | Follow-up time, months median (range) | Incidence of grade ≥ 3 late complication (%) | Surgical mortality (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
Small bowel obstructions | Fistula | Abscess | Wound dehiscence | Ureter | Bladder/urethra | |||||
Bosman et al. (2014), retrospective (1994–2013) | Diarrhoea 5 Neutropenic sepsis 1 | – | – | 9.9a | 15.9a | 9.9a | – | 10a | 4.6 | |
Ng et al. (2013), retrospective (1997–2008) | Skin 5 Gastrointestinal 9 Mucositis 2 | Termination 4 % | 15 (1–108) | 1.7b | 1.7b | 3.5b | 1.7b | – | 3.5b | 0 |
Sun et al. (2012), prospective (2004–2008) | Diarrhoea 10 Granulocytopenia 8 | Termination 4 % | 24 (10–57) | 1.4 | – | – | – | – | 5.6 | 0 |
Koom et al. (2012), retrospective (2000–2007) | Diarrhoea 9 | – | 14 | 5 | – | – | 27 | 14 | 0 | |
Das et al. (2010), retrospective (2001–2005) | Nausea/vomiting 4 | – | 25 (0–71) | 4 | 4 | 4 | 4 | 4 | – | 0 |
Milani et al. (2008), retrospective (2000–2005) | Gastrointestinal 12.5 | 0 | – | – | – | – | – | – | – | – |
Valentini et al. (2006), prospective (1997–2001) | Gastrointestinal 5 | Break 10 % | 36 (9–69) | 3 | – | – | – | – | 4 | 2.6 |
Termination 3 % | ||||||||||
A great proportion of late toxic events after multimodality treatment of LRRC is likely a consequence of surgery or local disease growth within the pelvis. It has already been said that the morbidity of surgery for LRRC can be as high as 60 %. Das and co-workers (2010) observed a trend towards a higher rate of grade 3–4 late toxicity in patients who had surgery than in patients who did not have surgery after reirradiation. More than half LRRCs re-recur or progress locally after treatment. In the series of Mohiuddin et al. (2002), small bowel obstruction without disease recurrence was seen in only 4 out of 15 (26.6 %) patients. Similarly in the series of Das et al. (2010), half of patients with small bowel obstruction also showed peritoneal carcinomatosis.
4.1.1 Prognostic Factors for Disease Control and Survival Outcomes After Long-Course Reirradiation
Several factors have been evaluated as potential prognostic determinants after reirradiation for LRRC. Response to chemo-reirradiation gives a better chance of achieving a R0 resection (Valentini et al. 2006).
A better local control was observed when R0 surgery was performed (Bosman et al. 2014; Valentini et al. 2006), when reirradiation doses higher than 30 Gy (Haddock et al. 2001) or 50 Gy10 (Koom et al. 2012) were delivered, or the interval between the primary treatment and recurrence was longer than 24 months (Valentini et al. 2006).
A better overall survival was observed in patients with good performance status (Karnofsky index ≥70), less-advanced stage of primary tumour (Mohiuddin et al. 2002), when the LRRC was completely resected (Bosman et al. 2014; Ng et al. 2013; Das et al. 2010; Valentini et al. 2006; Mohiuddin et al. 2002), when the interval between the primary treatment and recurrence was longer than 24 (Das et al. 2010) or 36 months (Bosman et al. 2014) or a reirradiation dose higher than 30 Gy was delivered (Mohiuddin et al. 2002).
4.1.2 Reirradiation Tolerance of the Pelvic Organs to Long-Course Reirradiation
Escalating the dose of reirradiation might improve the chance of local control and survival (Haddock et al. 2001; Mohiuddin et al. 2002; Koom et al. 2012). However, it is still unclear what the optimal dose of reirradiation is, since the tolerance of pelvic organs to reirradiation is poorly understood.
In the series of Bosman et al., there were no significant differences of incidence of acute toxicity between patients who were previously irradiated versus those who were not. This finding is in accordance with many clinical studies that have shown an almost complete recovery of acute responding tissue within a few months from irradiation (Langendijk et al. 2006; Würschmidt et al. 2008).
Much lesser is known about the reirradiation tolerance for late effects. The risk of late complications may depend on prior radiation dose. Das et al. (2010) observed a significantly higher incidence of late toxicity in patients who had received a radiation dose of ≥54 Gy than in those who had received a lower dose. The interval from the previous radiotherapy course might also have an impact. Long-term complications were reduced significantly in patients whose interval to reirradiation was longer than 24 months in the series of Mohiuddin et al. (2002).
Tumour location can be a predicting factor for toxicity risk too. In the series by Koom et al. (2012), patients with an axial or anterior tumour location had a significantly higher rate of grade 3 or 4 late toxicities than patients with a lateral or posterior tumour location (64 vs. 9 %).
Many of the published studies have employed hyperfractionated regimens in an attempt to minimize potential late toxicity. Mohiuddin et al. (2002) evaluated long-term results of reirradiation in 103 patients with recurrent rectal carcinoma. Patients were treated with either 1.8 Gy fractions daily or 1.2 Gy fractions twice daily, with a median dose of 34.8 Gy. Long-term complications were significantly reduced in patients receiving hyperfractionated radiation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree