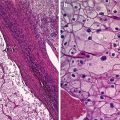
Fig. 1
Histology of a typical mature cystic ovarian teratoma. (a) Macroscopic findings show an unilocular cyst with typical content of sebaceous material and hairs. (b) Microscopic findings show a mature cystic teratoma lined by mature epidermis and subtended by connective tissue containing exuberant pilosebaceous follicles (arrow)
Typical Imaging Findings
Unlike US imaging for which MCT diagnosis could be difficult because of confusion with bowel or perforated appendicitis with appendicolith or ovarian carcinomas [19, 20], cross-sectional imaging (i.e. CT and MR) allows a relatively easy diagnosis because both of these modalities are extremely sensitive with respect to the presence of fat and calcifications (teeth), which represent the most frequent diagnostic findings in MCTs [21].
Most cases present as an asymptomatic adnexal mass incidentally detected on routine pelvic examination. If abdominal pain is present, the imaging findings show evidence of a complication such as MCT torsion, or else rupture must be sought.
The bilateral MCT involvement rate in the literature is 8–15 % (Fig. 2), and the mean diameter is reported to be around 7 cm [7]. The surface of a benign MCT is always smooth. Most are unilocular cysts and contain a large amount of fat in the form of liquid sebum, which is of low density on CT, with a high signal on MR images. Exceptional cases of solid mature teratomas have been reported in imaging studies [22, 23]. Fluid may fill the dependent portion of the tumour, producing a fat/fluid interface with the overlying liquid sebum. Layering or floating debris or both can also be encountered at this fat/fluid interface. Calcifications are commonly noted, but their presence does not indicate ovarian teratomas, and therefore, it is necessary to focus on detecting intratumoural fat in order to confirm the diagnosis. A nodular or palm tree-like mural protrusion, known as a Rokitansky protuberance or dermoid plug, is common. There is only one plug, which is usually rounded and typically contains fat, calcium or hair and a minority of diverse other tissues. Although these protuberances may be partly solid, they never show transmural growth (Fig. 3). Recently, intracystic floating balls were reported to be pathognomonic of mature cystic teratomas [24, 25] and were generated by sebaceous debris with skin squamae and hair [26]. These spherical structures are approximately 1–3 cm in diameter, and there may be more than 100 [27]. However, these teratomas usually have fat in their walls or in the Rokitansky nodule.
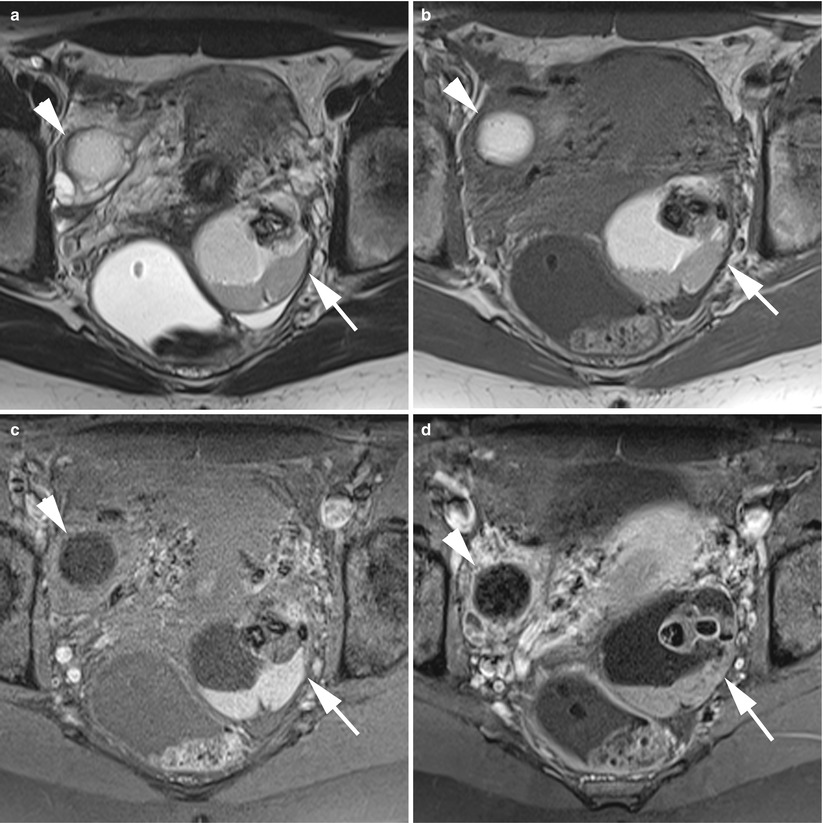
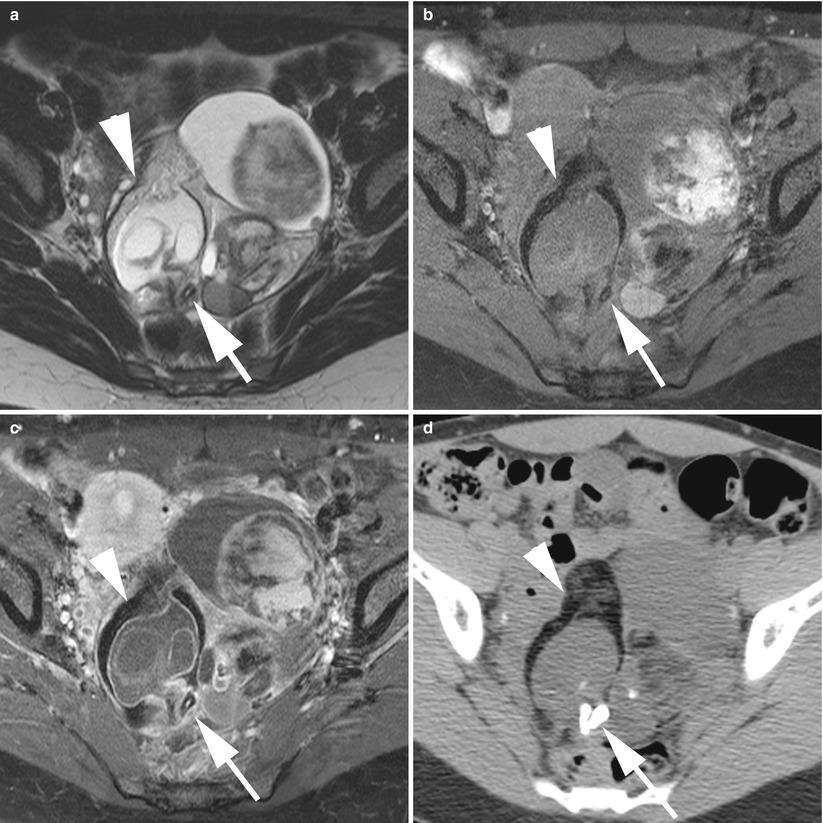

Fig. 2
MR of a bilateral ovarian teratoma in a 29-year-old woman. (a) Axial T2-weighted TSE, (b) axial T1-weighted GE, (c) axial fat-suppressed T1-weighted GE, (d) axial gadolinium-enhanced fat-suppressed T1-weighted GE. This patient was treated by bilateral cystectomy, and the final histological findings revealed a left cystic mature teratoma and a left monodermal teratoma. Note the typical appearance of the left one with a fat cystic component with hair level and the Rokitansky nodule with calcification and thin enhancement after contrast

Fig. 3
Imaging of a complex ovarian mature teratoma. (a) Axial T2-weighted TSE, (b) axial fat-suppressed T1-weighted GE, (c) axial gadolinium-enhanced fat-suppressed T1-weighted GE, (d) axial unenhanced CT scan (image at the same level as MR). Note the complex multicystic mass with areas of fat components (arrowheads) and teeth (arrows). The septa and the wall were enhanced after contrast but without extension beyond the capsule. The final histological findings revealed a cystic mature teratoma without malignancy
The identification of teeth or a Rokitansky protuberance or a large fatty component is a diagnostic sign of a benign cystic ovarian teratoma (cf. Table 1).
Table 1
Cross-sectional imaging findings of mature cystic teratomas
Imaging findings | |
|---|---|
Typical imaging | Unilocular |
<45 years | Intracystic fat |
<10 cm | Teeth/calcifications |
Fat/fluid level | |
No enhanced Rokitansky protuberance | |
Floating balls | |
Atypical imaging | Multilocular |
No fat in the cyst cavity | |
Small amount of fat in the wall or the Rokitansky nodule | |
Complex mass: raises the possibility of a collusive tumour, mixed germ cell or degenerescence | |
Suspicion of complication | Torsion: enlarged tubal mass, displacement of adnexa, deviation of uterus, pelvic fat infiltration, asymmetric smooth wall thickening |
Symptomatic with pain or fever | Rupture: flattened shape or discontinuity of the wall, ascites, omental infiltration, fatty implants, dense adhesions |
Malignancy (see below) | |
Infection: concomitant local or systemic infection, pelvic fat infiltration, thickened enhanced wall | |
Suspicion of malignancy | Highly enhanced Rokitansky nodules |
>45 years | Solid portion with moderate or intense enhancement |
>10 cm | Solid portion with transmural growth (outside the septa or the mass wall) |
Neighbouring organ invasion | |
Disseminated metastasis |
CT Imaging
Only a few original studies have reported CT imaging findings of benign MCT series [28–30]. The largest study includes 41 proven histological lesions and was published in 1989 by Buy et al. [17].
At CT, fat attenuation within an unilocular or multilocular ovarian cyst is a diagnostic sign of mature cystic teratomas and was found to be present in 84–93 % [17, 29] of cases. Fat is easily depicted, especially if its proportion is large (Fig. 4), thus allowing density measurement (typically found to be −20 UH or less). Sometimes, only a careful in-depth evaluation will reveal a fat-containing lesion when the fat proportion is millimetric, particularly when located in the wall or the Rokitansky nodule. A fat–fluid level is reported in 12 % of cases and teeth and other calcifications (even in the cyst wall) in 56–84 % of cases. Tufts of hair were common and present in 65 % of cases [17]. If hair is mixed with sebum, the lesion may have a radiologic density greater than that of fat (water density up to +8 UH [29]). There have been some reports on the presence of spherical structures (single or multiple) floating at the fat–aqueous fluid interface, this is the so-called floating mass which appears to be hypodense [31], typical of MCT. CT showed a Rokitansky protuberance in 21/23 (91 %) cases in Guinet’s series and in 35/43 (81 %) of cases in Buy’s series. A Rokitansky protuberance is defined as a solid or partially solid rounded (or bridge) structure, clearly linked to the cyst wall, projecting inside the cyst, or as a flattened (possibly irregular) wall thickening containing dense structures (calcified structures) and\or areas of fatty tissue [29].
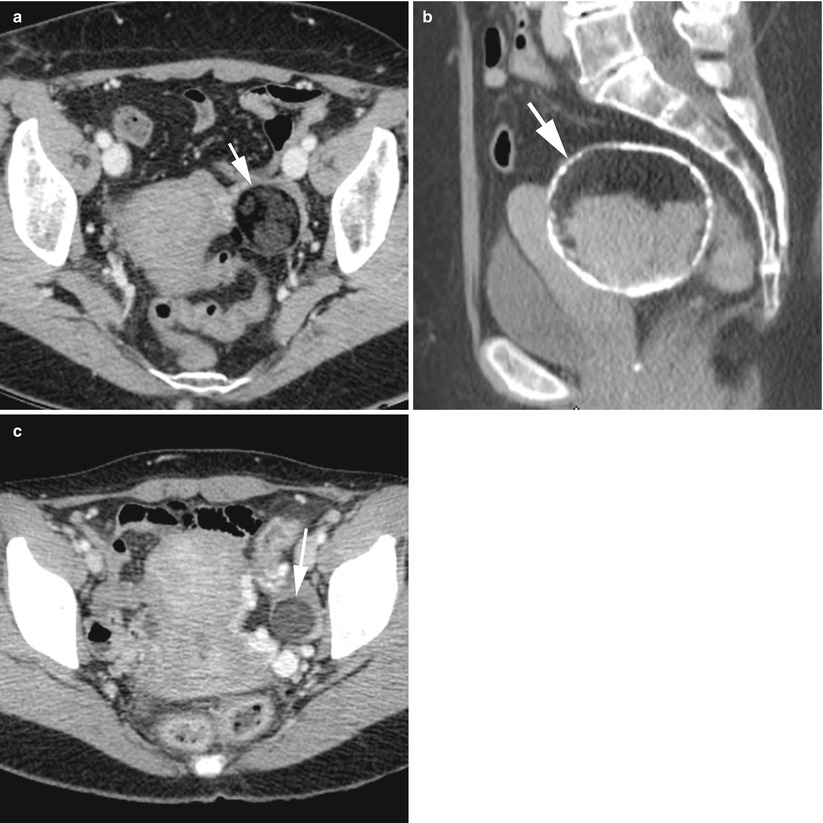

Fig. 4
CT of a left ovarian mature cystic teratoma (arrows) in three different women. (a–c) Axial contrast-enhanced CT scan (venous phase). Note the fatty component which is always predominant, easy to depict. Note also the thin calcifications in the wall of case “b”, which are rare; calcifications are usually found in the form of teeth
To date, the best suggestive patterns at CT include a fat-containing ovarian cyst and/or identification of a Rokitansky protuberance [29].
MR Imaging
At MR imaging, the sebaceous component of dermoid cysts has a very high signal intensity on T1-weighted images, similar to retroperitoneal fat. The signal intensity of the sebaceous component on T2-weighted images is variable, usually approximating that of fat (Fig. 5). Fat saturation techniques are essential in order to differentiate whether hyperintense areas in the tumours on T1-weighted images are due to haemorrhage, fat and/or fluid of high protein content. In MCT, fat is currently detected with spectral fat saturation, chemical shift imaging (in-opposed phase) or STIR (short T1 inversion recovery) sequences, with the first two being commonly used and chemical shift imaging being the most sensitive, especially to depict very small amounts of fat [32–34]. Intratumoural fat gives a high signal intensity on T1-weighted images and the signal drops on fat-saturated T1-weighted images. It is essential that images be obtained at the same level for each sequence in order to characterise each tissue component, and thus to be able to depict small amounts of fat. When used, chemical shift artefacts were encountered at the interface of fatty and non-fatty components. Fat may be seen in the cavity of the cyst or as a round mass floating at the water and fatty fluid interface, or only in the Rokitansky nodule. In addition, intracystic nondependent spheres of lipid material can occasionally be identified in an MCT, with a striking appearance [35].
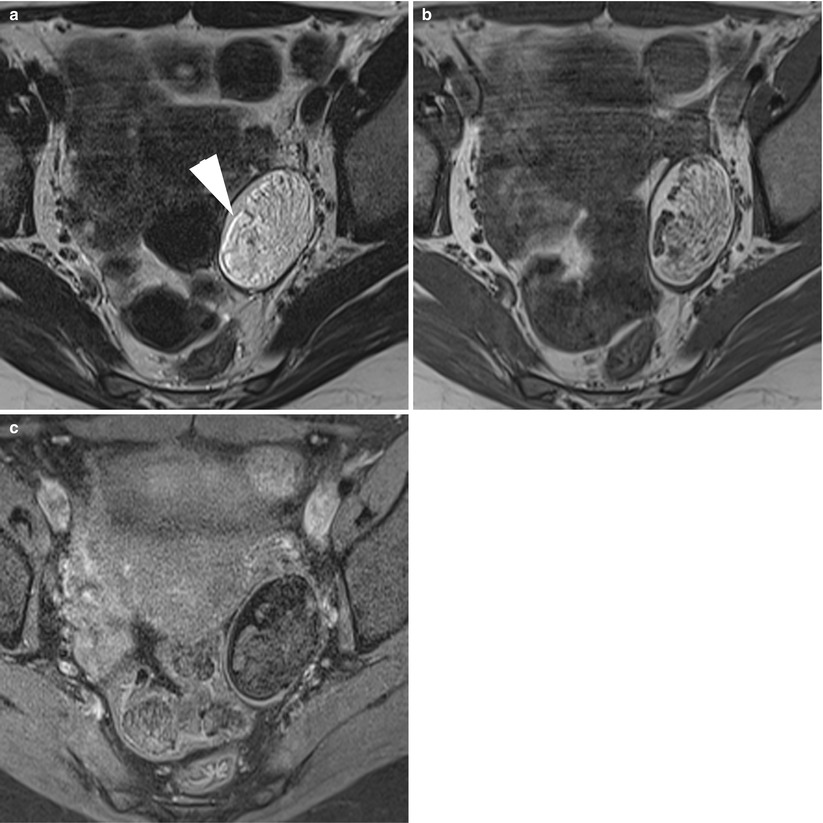

Fig. 5
MR of a typical left ovarian cystic mature teratoma. (a) Axial T2-weighted TSE, (b) axial T1-weighted GE, (c) axial fat-suppressed T1-weighted GE. Note the chemical artefact on the T2-weighted image (arrowhead) at the fatty–non-fatty interface
Calcifications and teeth both give a low signal intensity on T1 and T2-weighted images. Some mobile globules (so-called floating balls) can be encountered as spherical structures with a centre having a relatively low signal intensity on T1-weighted MR images compared with the outer portion, corresponding to hair or softer components [25] (Fig. 6). In T2-weighted images, the outer portion of the spherical structures are hypointense and the centre relatively hyperintense. The specific gravity of spherules or globules is lower than that of the surrounding fluid, so they float and are mobile in the cyst [26]. It has been postulated that each spherule is formed by the aggregation of sebaceous matter around a nidus (a tiny focus of debris, desquamative material or fine hair shafts) and may be formed within a discrete mass because of the difference in physical and thermal properties of the material being deposed [21].
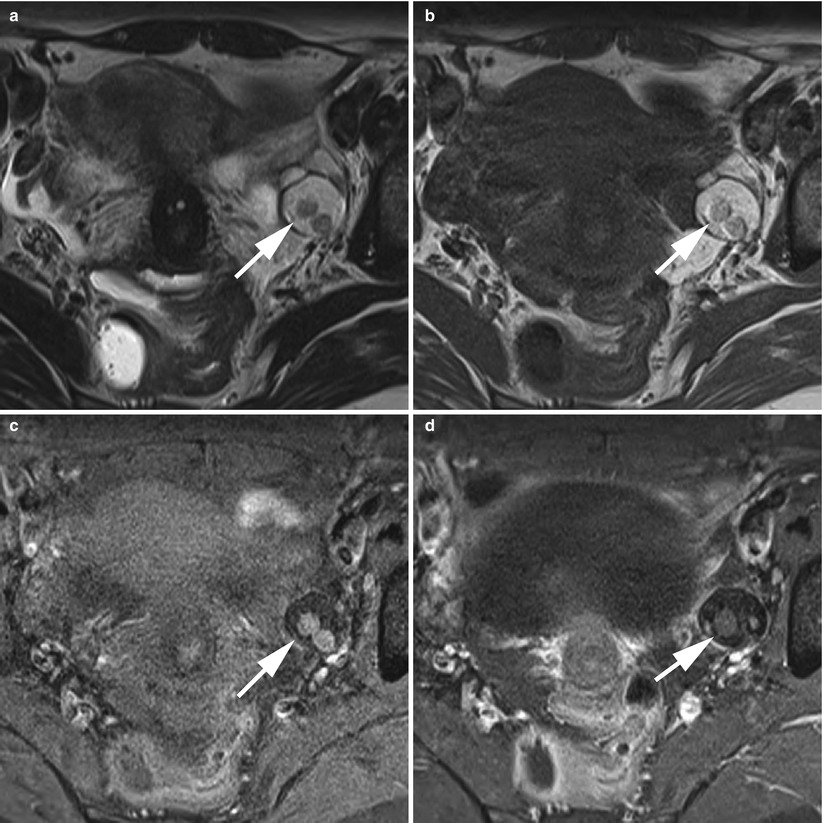

Fig. 6
Floating ball (arrows) in a left ovarian cystic teratoma in MRI. (a) Axial T2-weighted TSE, (b) axial T1-weighted GE, (c) axial fat-suppressed T1-weighted GE, (d) axial gadolinium-enhanced fat-suppressed T1-weighted GE
Recently, Nakayama et al. [36], showed that mature cystic teratomas tended to show significantly higher signal intensity on diffusion images and had areas of lower ADC (apparent diffusion coefficient) values than endometrial cysts and other benign and malignant neoplasms. This result was attributable to the keratinoid substance within the tumours (Fig. 7).
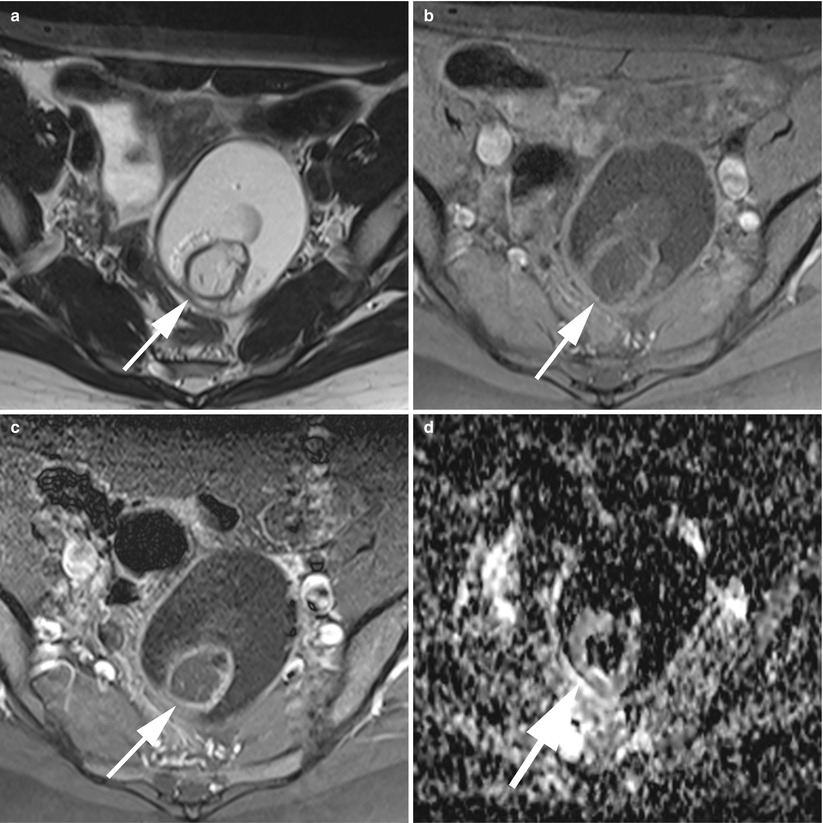

Fig. 7
MR of a typical ovarian cystic mature teratoma. (a) Axial T2-weighted TSE, (b) axial fat-suppressed T1-weighted GE, (c) axial gadolinium-enhanced fat-suppressed T1-weighted GE, (d) axial diffusion-weighted (ADC). This is a unilocular fat-containing cyst with a fatty Rokitansky protuberance (arrows). Note the very low ADC of the fatty component and the linear thin enhancement of the Rokitansky nodule wall
Atypical Aspect
Without Fat in the Cystic Cavity
A minor percentage (arising 15 % in a retrospective series of 78 of MCT in MRI [37]) of MCTs has only a small amount of fat or no visible fat in imaging studies. Small fat components must be carefully searched for in the cystic wall or in Rokitansky nodules [37] (Figs. 8 and 9). A fat-saturated MR imaging technique is advocated to improve diagnosis by showing small amounts of fat. A gradient echo technique with both in-phase and opposed-phase imaging seems better than the fat saturation method [21].
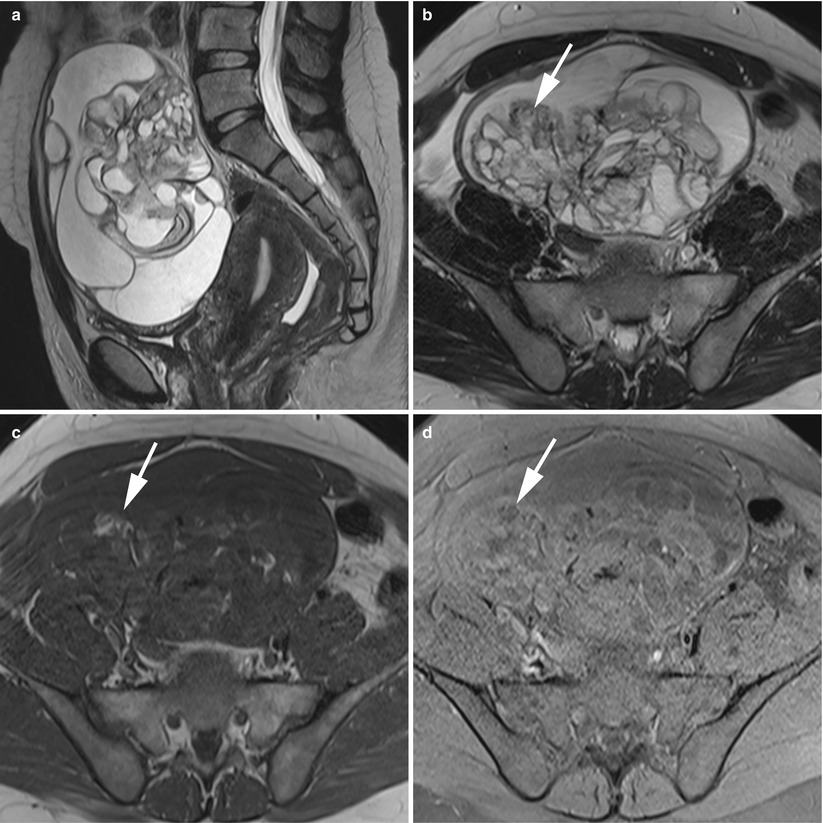
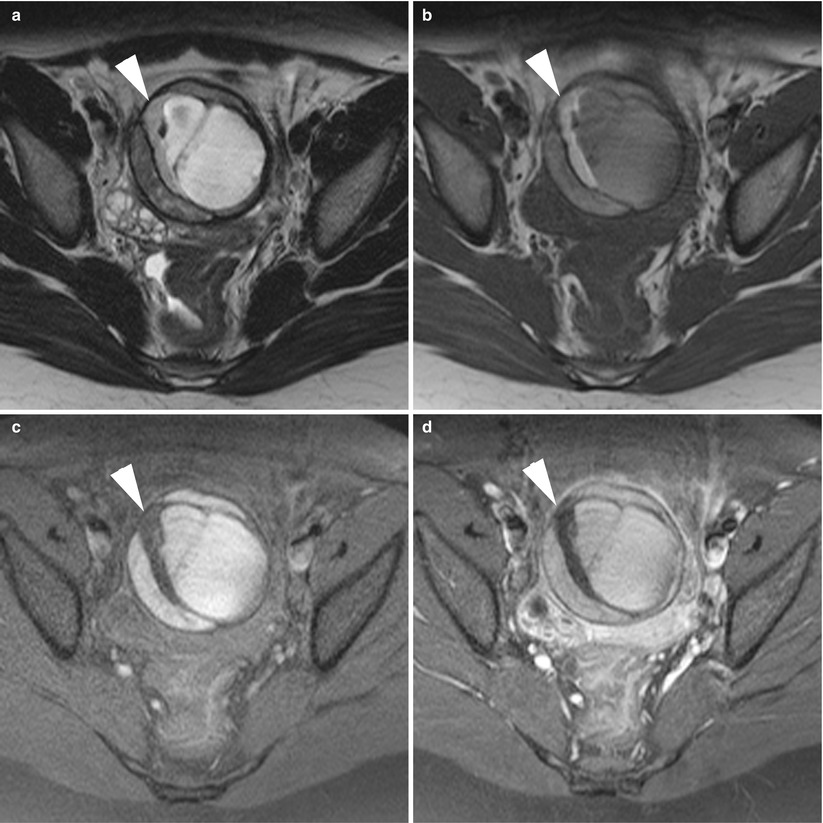


Fig. 8
MR of an atypical ovarian mature teratoma in a 19-year-old woman. (a) Sagittal T2-weighted TSE, (b) axial T2-weighted TSE, (c) axial T1-weighted GE, (d) axial fat-suppressed T1-weighted GE, (e) axial diffusion-weighted (b1000) (f) axial diffusion-weighted (ADC). Note the multicystic appearance of the mass, which corresponds to a multicystic Rokitansky nodule. Some of these loculi contain fat components (arrows) which appear with very low ADC values

Fig. 9
MR of a left atypical ovarian mature cystic teratoma in a 12-year-old girl. (a) Axial T2-weighted TSE, (b) axial T1-weighted GE, (c) axial fat-suppressed T1-weighted GE, (d) axial gadolinium-enhanced fat-suppressed T1-weighted GE. Note the atypical fatty component which is crescent-shaped (arrowheads) inside a multilocular lesion. The histological findings revealed simple mature cystic teratomas without neither malignancy nor mixed tumours
With a Pure fatty Component in the Cavity
These tumours are rare and may mimic other uncommon lipid-containing pelvic tumours (see section “Other lipid-containing pelvis tumours”).
Only Solid
This is a very rare finding, and these tumours are indistinguishable from other ovarian tumours, particularly with malignancy.
Collision Tumours
Collision tumours are tumours in which the different neoplastic components remain histologically distinct and separated from each other by narrow stromata or their respective basal laminae. There are various hypotheses concerning the formation of collision tumours. According to the first hypothesis, coexistence of two primary tumours in the same tissue is due to a “chance accidental meeting”. Secondly, it has been proposed that the presence of a first tumour alters the microenvironment and gives rise to the development of a second primary tumour or seeding of metastatic tumour cells. The third theory suggests that each primary tumour originates from a common stem cell. Concerning teratomas such an association had been reported with granulosa cell tumours [38], with serous cystadenocarcinomas [7, 39, 40] and more commonly with benign or malign mucinous tumours [41–43]. Mucinous tumours were found to coexist with 11.3 % of dermoid cysts, and dermoid cysts were found to coexist with 7.8 % of mucinous tumours [44]. Imaging studies of a collision tumour composed of a teratoma and a mucinous tumour show a typical multiloculated cystic mass with an internal loculi filled with pure fat (Fig. 10). The differential diagnosis was with mixed germ cell tumours, mixed epithelial tumours or malignant degeneration of cystic teratomas. Preoperative suggestion of a collision tumour is possible [41] and may lead the pathologist to perform an in-depth examination and take sections from suspicious areas, thus preventing underdiagnosis during intraoperative consultation for the tumour.
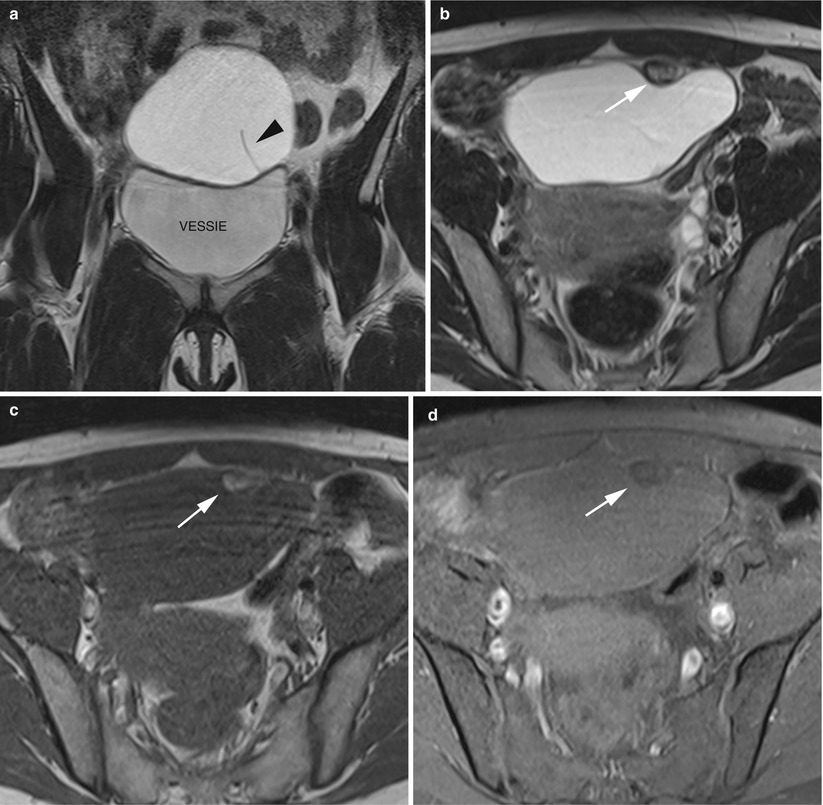

Fig. 10
MR of an ovarian collusive tumour in a 28-year-old woman. (a) Coronal T2-weighted turbo spin echo (TSE), (b) axial T2-weighted TSE, (c) axial T1-weighted gradient echo (GE), (d) axial fat-suppressed T1-weighted GE. The mass was predominantly cystic with thin septa (arrowhead). Note the small and unique loculi which contain fat components (arrows). The histological findings revealed an association of a mature teratoma and a mucinous cystadenoma
Mixed Germ Cell Tumours
A mixed tumour has intermixed varying histological components derived from a common stem cell. In the case of mixed germ cell tumours, virtually any combination of cell types can occur among embryonal carcinomas, dysgerminomas, teratomas and yolk sac tumours (endodermal sinus tumours). The imaging findings of mixed germ cell tumours are variable and reflect the diversity of this group of tumours. When a predominantly solid and heterogeneous ovarian tumour contains fatty areas or calcifications suggestive of a mature cystic teratoma or when a mature cystic teratoma contains an enhancing solid portion, a mixed germ cell tumour diagnosis should be considered.
Elevated serum α-fetoprotein (yolk sac tumour) and human chorionic gonadotropin (dysgerminome) levels and younger age can help in the diagnosis of mixed germ cell tumours [45].
Growing Teratoma Syndrome
Growing teratoma syndrome (GTS) is a rare finding, defined as an enlarging mature teratoma that arises during or following chemotherapy for a malignant germ cell tumour, especially an immature teratoma [46]. These tumours can undergo tissue maturation and take on an appearance more typical of mature cystic teratomas, a phenomenon also known as retroconversion [47]. Selective elimination of malignant cells by chemotherapeutic agents or differentiation of malignant cells into mature teratoma components following exposure to chemotherapeutic agents may be the two possible mechanisms responsible for GTS development. As a result, this is often misinterpreted as a chemoresistant tumour or recurrence [48]. These retroconverted masses can remain stable for a long period of time. By definition, GTS must exhibit normalisation of previously elevated tumour markers (alpha-fetoprotein [AFP] or beta human chorionic gonadotropin [HCG]), tumour enlargement or the presence of a new tumour mass and only mature teratoma elements in the pathologic examination. The radiologic features include increased mass density with well-circumscribed margins, onset of internal calcification with fatty areas and cystic changes [49].
Complications
MCT complications include torsion (16 %), malignant degeneration (2 %), rupture (1–2 %) and infection (1 %) [50].
Torsion
Torsion is the most common complication associated with mature cystic teratomas. The torsion rate was reported at 3.2–16 % [8]. From another standpoint, about 30 % of ovarian torsions are due to mature cystic teratomas which, along with serous cystadenomas, represent the most common cause of ovarian torsion [51]. The classical clinical presentation includes sharp, localised right or left lower abdominal pain, tenderness, peritoneal findings and a pelvic mass. Gastrointestinal complaints with nausea and vomiting are encountered in approximately two-thirds of patients, whereas fever is an argument in favour of necrosis complicating ovary torsion.
The cross-sectional imaging diagnosis includes visualisation of a fat-containing adnexal mass in association with several signs of torsion, as listed below [52]:
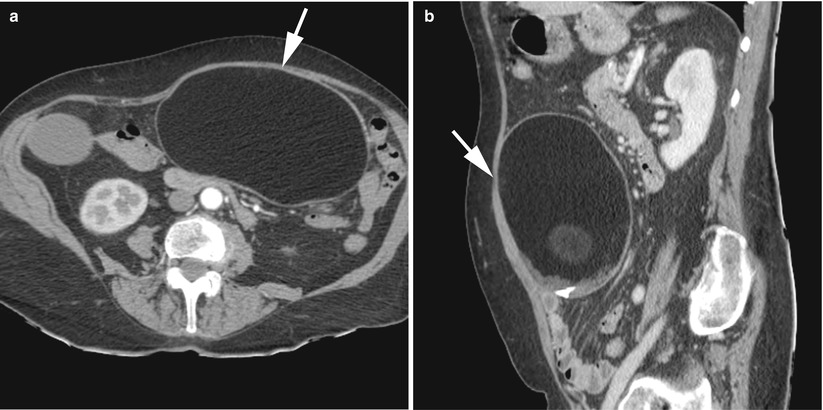
The presence of an enlarged solid tubal mass seen between the uterus and the ovarian mass (Fig. 11), which is the most specific imaging finding for adnexal torsion; it manifests as an amorphous or tubular mass-like structure or has a target-like appearance between the torsed teratoma and the uterus or a beaklike protrusion extending from the uterus and partially covering the ovarian teratoma [53].
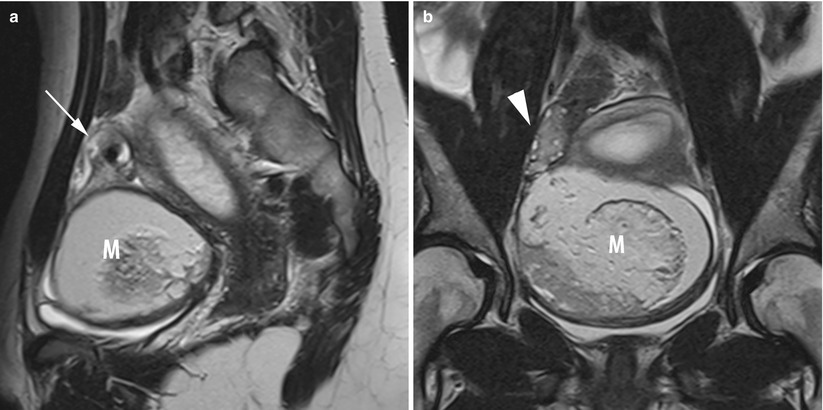
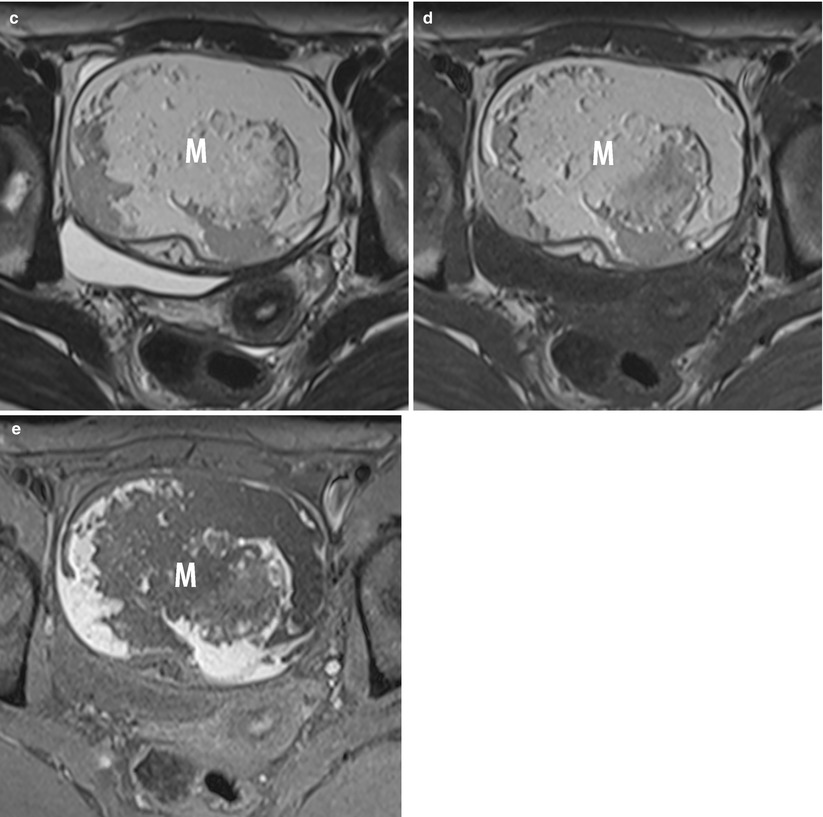
Fig. 11
MR of a left ovarian cystic teratoma torsion in a pregnant woman. (a–c) Respectively sagittal, coronal and axial T2-weighted TSE, (d) axial T1-weighted GE, (e) axial fat-suppressed T1-weighted GE. Note the normal right ovary (arrowhead), the spire of the torsed vascular pedicle (arrow) and the cystic teratoma (M). This patient was treated by total ovariectomy
The identification of a smooth wall thickening without nodularity on the cystic mass, this thickening is more often eccentric and >1 cm [54] than concentric.
The displacement of adnexa which may be on the contralateral side of the pelvis or on the midline in a far anterior position abutting the anteropelvic fascia or posterior in the pouch of Douglas (Fig. 12).
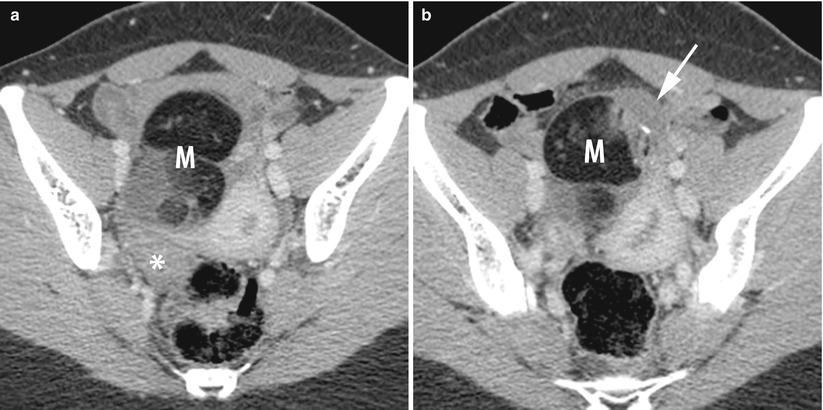
Fig. 12
CT of a left ovarian cystic teratoma torsion. (a, b) Axial contrast-enhanced CT scan (venous phase). Note the normal right ovary (star), the typical left ovarian cystic teratoma with a fat component (M), the uterine displacement on the twisted (i.e. left) ovary and the thickened tubal mass (arrow) between the uterus and the teratoma
The deviation of uterus and the pelvic space infiltration on the side of a twisted ovary.
Mature cystic teratomas affected by torsion are larger than average (mean diameter, 11 cm versus 6 cm); this enlargement could be the result of torsion rather than the cause of it. Torsion does not eradicate the fatty components.
Degenerescence
Malignant transformation occurs in 0.17–2 % of cases [55] and may occur in any of the three germ cell layers, including the ectoderm, mesoderm and endoderm. The epithelial component of mature cystic teratomas commonly transforms into squamous cell carcinomas (more than 80 % of cases [56]) although other neoplasms, including adenocarcinomas [57], low-grade carcinoid tumours [58], neuroendodermal tumours, malignant melanomas [59], oligodendrogliomas [60], thyroid papillary carcinomas [61] and highly aggressive sarcomas [62, 63], have been reported. Most cases are discovered as incidental microscopic foci of squamous cell carcinomas in benign-appearing gross specimens of cystic teratomas.
Clinically, malignant transformation cannot be readily identified. Malignant transformation has been mainly found in women of over 50 years old, with high concentrations of squamous-cell-carcinoma antigen (SCC) and cancer antigen CA125 and with ovarian tumours more than 100 mm in size [4].
In imaging, the features reported in favour of malignancy are [30, 55]:
A diameter larger than 10 cm
The presence of an enhancing soft tissue mass component, especially contrast enhancement of the Rokitansky protuberance, which suggests the possibility of malignant transformation [56]
An obtuse angle between the enhanced soft tissue mass and the inner cyst wall
Areas of haemorrhage and necrosis
An extracapsular tumour growth with extension into adjacent structures
A disseminated metastasis
Direct invasion of adjacent tissue is common for squamous cell carcinomas arising in ovaries as well as in other parts of the body. Conversely, the most important mode of spreading in common epithelial ovarian carcinoma is dissemination by lymphatic spreading and peritoneal carcinomatosis. Consequently, an invasive growth pattern in an ovarian mass is an argument in favour of a degenerated mature cystic teratoma with squamous cell carcinoma.
Mori et al. reported that the combination of the patient’s age (>40 years) and serum SCC antigen level (>2.5 ng/mL) was 77 % sensitive and 96 % specific for malignant transformation [64].
The malignant teratoma prognosis is good if the tumour is completely excised and does not extend beyond the capsule [65]. Hence, a surgical approach (laparotomy versus laparoscopy) should be chosen carefully and based on the results of clinical and imaging investigations as well as tumour marker profiles. Complete resection together with hysterectomy, bilateral salpingo-oophorectomy and lymphadenectomy for patients with advanced disease, followed by adjuvant chemotherapy with an alkylating drug, was associated with higher survival, whereas radiotherapy was not [4].
Rupture
There is a low rate of spontaneous rupture of mature cystic teratomas (1.2–3.8 %) because of the usually thick capsule. It causes leakage of the liquified sebaceous contents into the peritoneum and leads to either acute or chronic clinical presentations [66], and the latter is the most common.
Acute Chemic Peritonitis
This is caused by the sudden rupture of tumour contents, which may occur spontaneously or more commonly in association with torsion, trauma, infection or labour [67]. On imaging, detection of a flattened shape or discontinuity of the tumour wall is a sign of a ruptured teratoma and can present with ascites and diffuse or focal omental infiltration.
Chronic Granulomatous Peritonitis
This results from a chronically leaking dermoid cyst, which can be characterised by multiple small white peritoneal implants, dense adhesions and variable ascites that simulate carcinomatosis or tuberculous peritonitis. Sometimes, fatty implants may be identified within the peritoneal cavity which are diagnostic signs in this case [68].
Furthermore, in addition to rupture into the peritoneal cavity, perforation into other intra-abdominal organs has been reported, including rupture into the bladder, small bowel, rectum, sigmoid colon, vagina and even through the abdominal wall.
Another entity has been described, which is referred to as a “parasitic ovarian dermoid tumour” that could occur when an ovarian dermoid tumour has undergone autoamputation (total or semi) and reimplantation on another abdominal site [69]. The most common is reported in the greater omentum (Fig. 13), but other sites have been described, such as within an indirect inguinal hernia sac [70] or within an extensive intra-abdominal parasitic form [71]. Autoamputation could be inaugural (the so-called parasitism) or secondary to torsion or surgical rupture. Another theory is that primary teratomas of the omentum originate from displaced germ cells. They could also develop in congenital omental supernumerary ovaries. At imaging, it appeared like an ovarian mature cystic teratoma with a fat component at an ectopic location.